CE Approved Disposable Sample Release Kit
Qingdao Toyo Industry Co., Ltd. / 2022-06-23
- Type:Test Strips & Test Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult & Children
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:RJ-C
- Function:Virus Samples for Storing & Transportation
- Sterile:EOS
- MOQ:1000
- Free Samples:Available
- Transport Package:Carton
- Specification:25 Kits,Box
- Trademark:Renji
- Origin:China
- HS Code:3822009000
- Production Capacity:100000000
=== Description ===
50kits/box,16 box/carton Box size:225x145x165mm weight:0.7KG Carton size:600x480x350mm Weight:12.5KG
Bulk Package:
50kits/box,20 box/carton Box size :225x120x165mm
weight :0.65KG Carton size :620x470x350mm weight:14.5KG

Intended Use
We are specialized in the medical equipment industry ,Our disposable virus specimen collection tubeare used for the collection and transportation of clinical, Influenza, Avian Influenza, Hand, Foot and Mouth Disease, Measles, Chlamydia, Mycoplasma, and Urea plasma specimens.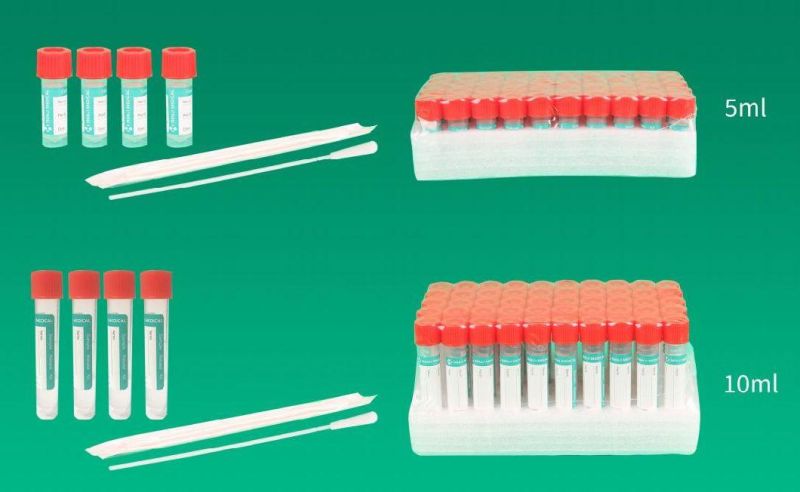
Kit Features
- Saliva Kit
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





