Lab Test Sterile Polypropylene 96 Well Cell Culture Multi-Well Plates
Brilliant Tin Box Manufacturing Co., Ltd. / 2022-06-23
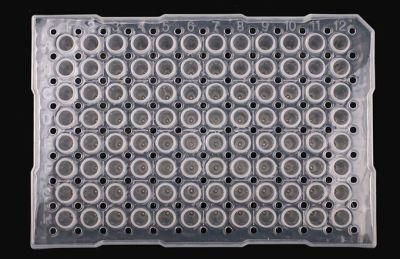
- Type:96 Well Cell Culture Multi-Well Plates
- Material:Plastic
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:All
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:RM-698
- Transport Package:100PCS,Carton
- Specification:215*30mm
- Trademark:Runmei
- Origin:China
- Production Capacity:50000000PCS Per Month
=== Description ===
Basic Info.
Model NO. RM-698 Transport Package 100PCS/Carton Specification 215*30mm Trademark Runmei Origin China Production Capacity 50000000PCS Per MonthProduct Description
Lab Test Sterile Polypropylene 96 Well Cell Culture Multi-Well Plates

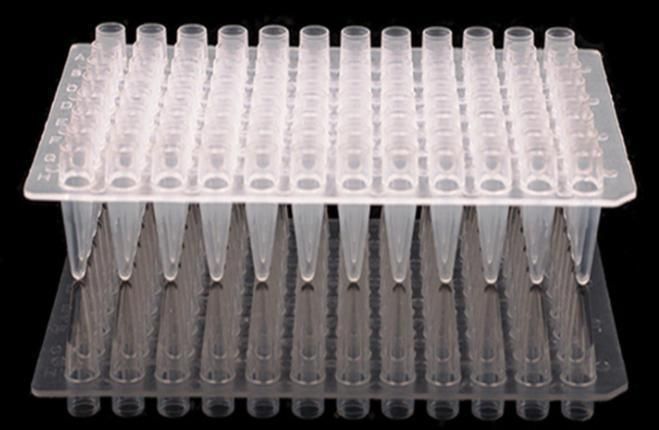 Wide range of applications, such as agglutination and enzyme-linked immunosorbent assay experiments (U-shaped bottom), centrifugation and sedimentation (V-shaped bottom), colorimetric assay and suspension cell culture (F-shaped bottom)
Wide range of applications, such as agglutination and enzyme-linked immunosorbent assay experiments (U-shaped bottom), centrifugation and sedimentation (V-shaped bottom), colorimetric assay and suspension cell culture (F-shaped bottom)Size conforms to ANSI/SBS microplate standard, compatible with standard type and automation equipment
Features:
· High light transmittance, low background interference
· Number-letter coding, easy to identify
· The directional locking lid can minimize water vapor evaporation caused by long-term cultivation
· Stackable

Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the development of the gene detection products and the construction of big data service platforms led by a team of doctors at home and abroad. Our company's strategic goal is to base on China and radiate the world, and to solve the pain points and difficulties of the industry and create value for human beings as our corporate purpose. At present, our company has completed the construction of product systems for pathogen biology fluoresence quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology immune colloidal gold detection kits. Our company has completed the construction of the national pathogenic biology product and service marketing network and the development of overseas detection product markets. We have a professional technical team, advanced manangement mode and perfect business operation mechanism. We are focusing on IVD detection kits. We have our own R&D team, manufacture, many of them are professional doctors who have plenty of experience in this industry. Our products have approved many certificates like ISO, SGS,CE,CFDA and so on.

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





