Exsanguinating Device Rubber Disposable Latex Free Tourniquet Surgical Hemostatic First Aid Tourniquet Surgical Tourniquet Supply in China
Yiwu Bokun Adhesive Tape Co., Ltd. / 2022-06-23

- Customized:Non-Customized
- Certification:ISO, CE
- Feature:Disposable
- Color:Red
- Type:Rubber Tourniquet
- Application:Limb Surgical
=== Base Info ===
- Model NO.:M
- Sterilized or Not:Yes
- Sterilization Method:Ethylene Oxide (Eto)
- Shelf Life:3 Years
- Reused or Not:No
- Model:Upper Limb and Lower Limb
- Suitable:Surgical Hemostatic
- Transport Package:Box
- Specification:M
- Trademark:E. R. A
- Origin:China
- Production Capacity:500, 000pieces,Year
=== Description ===
BLOD Exsanguinating Tourniquet Ring VS Tourniquet Pneumatic Tourniquet
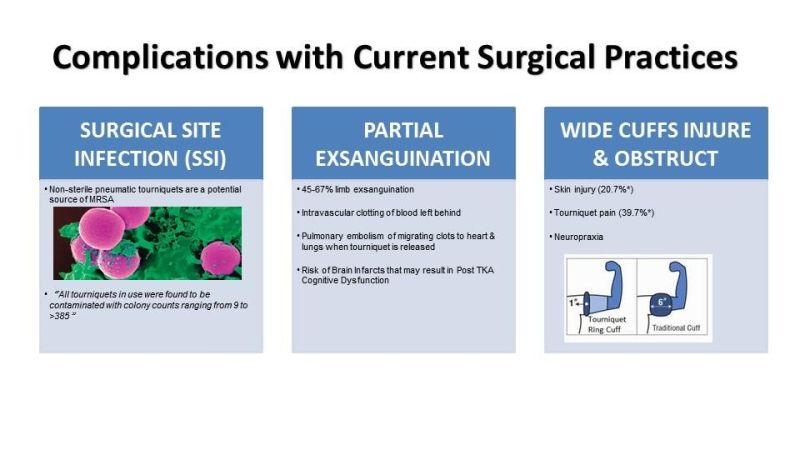
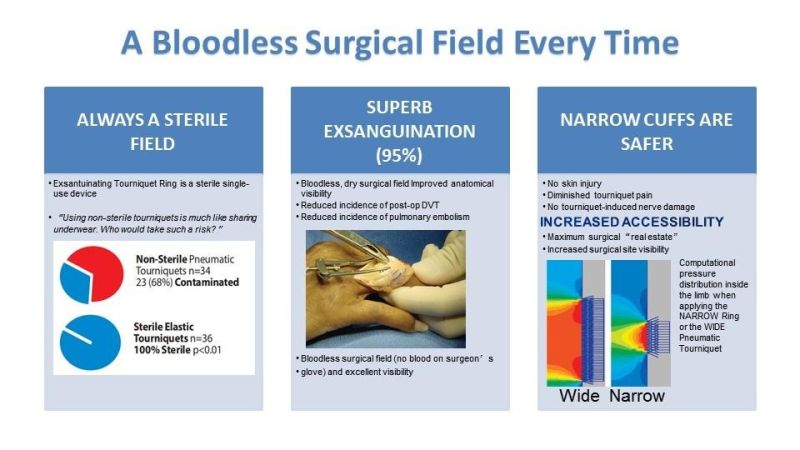 Company Details:
Company Details: 
Our company established in the year of 2007 start with the business of chemicals, and the market cover Europe, North America of Canada, USA, Latin America, South America, Africa, South East Asia...more than 50 countries and areas.
The turnover reached 50 million USD with more than 10 years hard working and developed on the year of 2020.
We explore in the field of medical from the year of 2019, including medical consumables, medical instruments,ect.
Believe that we will grow into an excellent enterprise in this field in the near future, and we are willing to be your window and bridge of communication in China!!!
Packaging &Shipping:


FAQ:
1. Can it be reused?
No. it is a sterile single-use device that is removed by cutting when the procedure ends. Therefore, it cannot be re-used or re-sterilized
2. How many units have been used worldwide?
At present, around 1 million units have been used in orthopedic procedures globally.
3. Is it Latex-free?
Yes.
4. How should it be stored?
The blod should be stored in a clean environment suitable for sterile medical products under the following conditions
- Temperature range: 7-28 degrees C (45-83 degrees F)
- Humidity: <60% RH
3 years
6. What happens after the expiration date has passed?
- Do not use if the expiration date has passed.
- After 3 years, the sterility of the device may be compromised.
- For additional instructions regarding expired devices, please contact us.
It is sterilized with Ethylene Oxide (EtO).
8. Do the materials that is composed of contribute to its sterility?
- It components (silicon ring, stockinet, and pulling straps) are non-sterile prior to assembly, after which the undergoes a process of sterilization.
- The product is shipped as a sterile unit.
Yes. The inside of the silicone ring is sterile as is the entire device.
10. What are contraindications for the Ring? Can the it be used on an infected limb or on patients with bone tumors?
No. Please read the following Contraindications before using
- Do not use it on patients with poor peripheral blood flow, edema, or Deep Vein Thrombosis (DVT ). See the Wells Score System for likelihood of DVT.
- Do not use if the limb is infected or with malignancy.
- Do not apply directly on skin that is fragile or has significant lesions.
- Use a sterile ace bandage to protect fragile skin before applying.
- Do not leave on a patient's limb for more than 120 minutes.
- Do not place it directly over the ulnar nerve (at the elbow) or the peroneal nerve (at the proximal tibia).
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





