Wholesale CE ISO Approved Helicobactor Pylori Antibody Test
Zhangjiagang City Nancheng Machinery Co., Ltd. / 2022-06-23

- Type:Test Strips & Test Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:RI412C
- Transport Package:Cartons
- Specification:63*41*37cm
- Trademark:EUGENE
- Origin:China
- HS Code:3002190090
- Production Capacity:100, 000,Day
=== Description ===
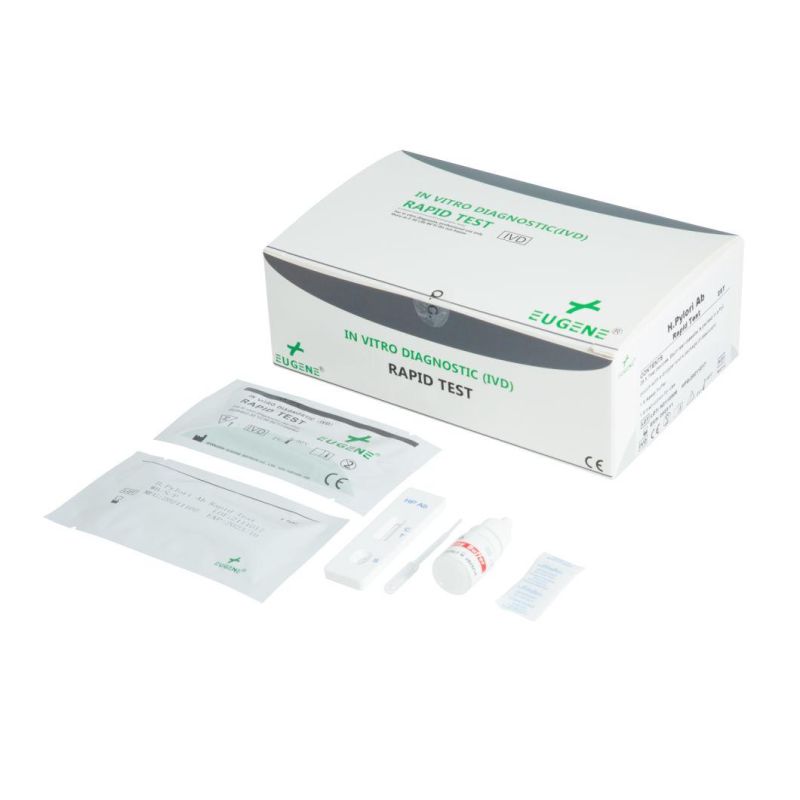

A lateral flow immunochromatographic assay for the qualitative detection of antibodies to Helicobacter Pylori (H. Pylori Ab) in human whole blood, serum or plasma.
The EUGENE® H. Pylori Ab Rapid Test is a lateral flow immumochromatographic assay which uses recombinant antigens to detect the antibodies (IgG, IgM, and IgA) to H. Pylori in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Helicobacter Pylori (H. Pylori). The test is recommended for professional use only. All results must be interpreted together with other clinical information available to the physician.
The EUGENE® H. Pylori Ab Rapid Test is a lateral flow immunochromatographic assay using recombinant antigens to detect the antibodies (IgG, IgM, and IgA) to H. Pylori in human serum or plasma. The membrane strip of the test cassette consists of: 1) a conjugate pad (sample pad); 2) a nitrate membrane strip containing a control line (C) and a test line (T). Both lines are not visible before performing the assay; When a sufficient specimen is added into the sample well (s), it will migrate by capillary action across the membrane strip. If increased level of antibodies (IgG, IgM, and IgA) to H. Pylori is present in the specimen, within the test window, a pink colored conjugated immunocomplex formed test line (T) will appear and it indicates a positive result. If the test line (T) does not appear, it indicates a negative result. The test contains an internal control line (C) which should appear as a red line regardless of color development on the test line (T). Otherwise, the test result is invalid and the assay shall be repeated with another test device.
INTERPRETATION OF RESULTS
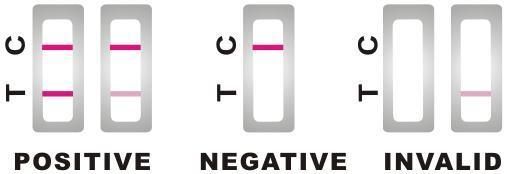 NEGATIVE: Only the red control line (C) appears. It indicates that no detectable antibodies to H. Pylori are present in the specimen. The result is negative.
NEGATIVE: Only the red control line (C) appears. It indicates that no detectable antibodies to H. Pylori are present in the specimen. The result is negative.POSITIVE: Two red lines appear, the control line (C) and the test line (T). It indicates that the antibodies to H. Pylori in the specimen are above the normal level. The result is positive.
The intensity of the test line ("T") may be less than that of the control line ("C"); this still means positive result.
Samples with positive results should be confirmed with alternative testing method(s) and clinical findings before a positive determination is made.
INVALID: If no red line appears in the position of control line (C), the test result is invalid regardless of color on the test line (T). Review the procedure and repeat the test with a new test device. If the problem persists, contact your local distributor.



New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





