PVDF Sutures for Cardiovascular Surgery OEM
Safecare Medical Products Co., Ltd.(Hefei) / 2022-06-23

- Type:Surgical Supplies Materials
- Material:PVDF
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:All
- Logo Printing:Our Brand or OEM
=== Base Info ===
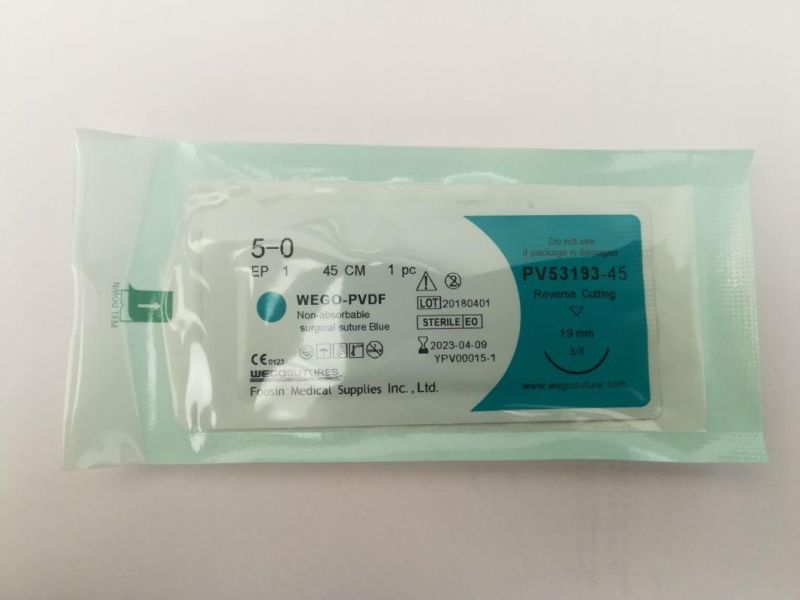
- Model NO.:USP 5,0
- Color:Blue
- 120pieces/Box:1200 Pieces,Carton
- Transport Package:1200pieces,Carton
- Specification:USP 12#-6,0
- Trademark:wegosutures
- Origin:China
- HS Code:9018322000
- Production Capacity:500000pieces,Year
=== Description ===
Basic Info.
Model NO. USP 5/0 Color Blue 120pieces/Box 1200 Pieces/Carton Transport Package 1200pieces/Carton Specification USP 12#-6/0 Trademark wegosutures Origin China HS Code 9018322000 Production Capacity 500000pieces/YearProduct Description
WEGOSUTURES already obtain CE mark and USA FDA 510K. WEGOSUTURES conformity with the USP and EP standard. suture needle Products including Braided PGA, Braided PGLA, Braided Rapid PGA, Monofilament PDO, Silk, Nylon, Polyester and Polypropylene. WEGOSUTURE if soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137.Mainly markets are US and European markets.? Over 6 years exported experience and obtained good reputation from customers.
our sutures products covers the full range of sutures sizes: from USP 7# to USP 12/0 including varies type of needles or non-needle products.
Sutures type including absorbable and non absorbable.
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





