Factory Price Respiratory Virus Rapid Antibody (IgG/IgM) Diagnostic Kit Test Kit
ZHENGZHOU KING PACK LONG MACHINERY CO., LTD. / 2022-06-23

- Type:Diagbostic Kit
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:One Year
- Group:All Persons
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:A
- Category:Diagnostic Consumables
- Single Use:Yes
- Sterile:Yes
- Operation:Simple and Rapid
- Price:Competitive
- Delivery Time:Fast
- Accuracy:High
- Place of Use:Both Home and Hospital
- Quality:Excellent
- Transport Package:Packed in Box, Shipped by Sea, Air and Express
- Trademark:OEM or ODM
- Origin:China
- Production Capacity:10000000000000sets,Year
=== Description ===
One test per bag for one person, 20 tests/kit
The product is intended for the qualitative detection of antibody content against Infectious Disease/virus in clinical samples (serum/plasma/whole blood).
Infectious virus, as a large virus family, is a single positive stranded RNA virus with envelope. The virus is known to cause major illnesses such as colds, Middle East Respiratory Syndrome (MERS), and Severe Acute Respiratory Syndrome (SARS). The core protein of the virus is the N protein (nucleocapsid), which is a protein component located inside the virus. It is relatively conserved among β-coronaviruses and is often used as a tool for the diagnosis of coronaviruses. ACE2, as a key receptor to enter cells, is of great significance for the research of viral infection mechanism.
The product is based on the principle of antigen-antibody reaction and immunoassay technique. The test device contains colloidal gold labeled recombinant protein, mouse-anti human IgG antibody immobilized in T2 test area, mouse-anti human IgM antibody immobilized in T1 test area and the corresponding antibody in quality control area (C).
During the test, when the IgM antibody level in the sample is at or above the limit of detection of the test, the IgM antibody in the sample binds to the colloidal gold recombinant protein which is pre-coated on a gold label pad. The conjugates migrate upward through capillary effect and would be captured by mouse-anti human IgM antibody immobilized in T1 test area subsequently and this produces a purple-red band appears in the T1 test area. When the IgG antibody level in the sample is at or above the limit of detection of the test, the IgG antibody in the sample binds to the colloidal gold recombinant protein which is pre-coated on a gold label pad. The conjugates migrate upward through capillary effect and would be captured by mouse-anti human IgG antibody immobilized in T2 test area subsequently and this produces a purple-red band appears in the T2 test area. If it is a negative sample, there is not a purple-red band appeared in the T1 and T2 test area. Regardless of the presence or absence of the antibody in the sample, a purple-red band will appear in the quality control area (C). The purple-red band in the quality control area (C) is a criterion for judging whether there is enough sample and whether the chromatography process is normal. It also serves as the internal control standard for reagents.
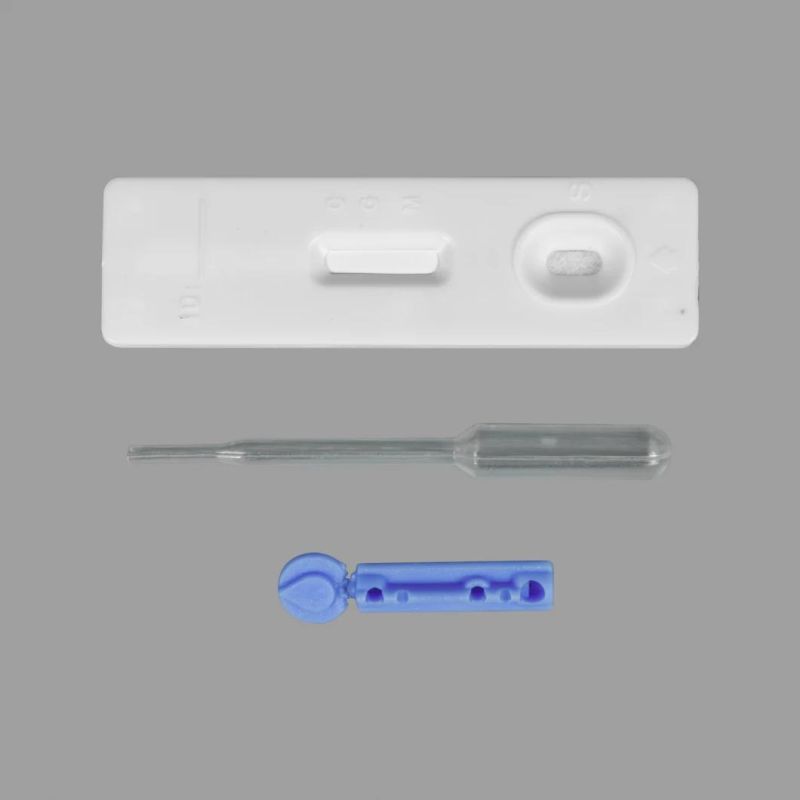
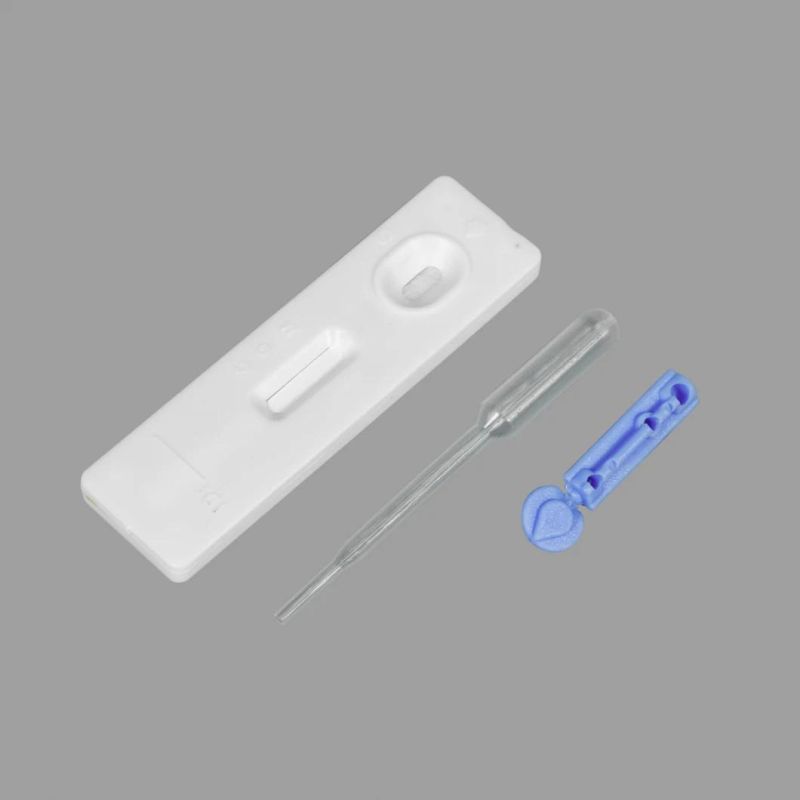
The product contains 20 tests, one IFU (instruction for use) and one lot number card.
For each test, it contains one testing strip, one dropper and one package of desiccant.
The testing strip is composed of one gold standard mat (colloidal gold recombinant protein), sample mat, cellulose nitrate membrane (Mouse-anti human IgM antibody immobilized in T1 area, Mouse-anti human IgG antibody immobilized in T2 area; Goat anti-mouse antibody immobilized in C area), absorbing paper, plastic carrier board.
It should be stored at 4ºC~ 30ºC, be kept dry and away from sunlight. The shelf life is 12 months.
For per test strip, it should be used within 1 hour after unsealing.
Production Date and Expiration date are shown in the package label.
The test strip can be performed with serum/plasma/whole blood.
The blood should be collected by professional medical staff, and it is advised of detecting serum/plasma in priority, and under emergency conditions or special conditions, the whole blood of patients can be used for rapid testing.
After collection of samples, it should be tested immediately. It is forbidden for long time placement of the sample under room temperature. For whole blood sample, if it can not be tested in time, it can preserve for 24 hours between 2 and 8ºC. Serum/plasma samples can be preserved for 3 days under temperature between 2 and 8ºC, and for long time storage, they should be stored under -20ºC, and it should avoided repeated freeze-thaw cycles.
Before testing, the sample must be restored to room temperature, ready for application only after homogeneity.
The sample must be returned to room temperature before testing, and should be used after mixing.
Do not use samples with severe hemolysis, severe lipids, and jaundice.
Please read the instruction for use carefully before performing the test. Before testing, restore the reagents and blood sample to room temperature.
1. Remove the test strip from the packaging reagent bag and use it within 1 hour, especially in an environment with room temperature higher than 30 ° C or in high humidity.
2. Place the kit on a clean platform.
l Serum or plasma sample: Add 10 uL of serum or plasma sample to well A, and then add two drops (about 80 uL) of sample dilution to well B, and start timing.
l Whole blood sample: Add 20 uL of whole blood sample to sample well A, and then add two drops (about 80 uL) of sample dilution to sample well B, and start timing.
3. Wait for the fuchsia band to appear. The test results should be read within 10-20 minutes. Do not read the results after 20 minutes.
l Positive (+): There appear purple stripes in both quality control area and either area T1 or T2.
l Negative (-): There is only one purple stripe in the quality control area (C), and without purple stripe in either test area T1 and test area T2.
l Invalid: There is no purple stripe in the quality control area (C), indicating incorrect operating procedures or the testing strip has already deteriorated. Under this conditions, it must read the instruction for use again carefully, and then use the new test strips to test again. If the problem still exists, stop using this lot number immediately and contact the local suppliers.
1. The test results of this product should be comprehensively judged by the physician in combination with other clinical information, and should not be used as the only criterion;
2. The product is used to test the respiratory virus antibody of the tested sample.


Company Profile

SHANGHAI TEAMSTAND CORPORATION is a professional supplier of medical products and solutions. "For your health", deeply rooted in everyone's hearts of our team, we focus on medical consumables and equipment, rehabilitation consumables and equipment, laboratory products, etc.
With over 10 years' experience in medical industry, we have exported to USA, EU, Middle East, Southeast Asia and other countries. And we have gained good reputation among all of these customers for good service and competitive price.
Headquartered in Shanghai, the biggest and modernized city in China, TEAMSTAND invests 2 factories in Shandong and Jiangsu, and cooperates with over 20 factories in China. "Top 10 medical supplier in China" is our goal, and we are closing to achieve this day by day.
Welcome all friends and customers all over the world in medical industry to contact us!
FAQ
1. Who are we?
We are based in Shanghai, China, start from 2019,sell to North America(60.00%),Oceania(15.00%),South America(5.00%) ,
EasternAsia(5.00%),Mid East(4.00%),Southeast Asia(2.00%),Western Europe(2.00%),Eastern Europe(2.00%),Northern
Europe(2.00%),CentralAmerica(1.00%),South Asia(1.00%),Southern Europe(1.00%). There are total about 5-10 people in our office.
2. How can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.What can you buy from us?
virus collection kit,auto-lock safety syringe,saliva collection kit,infusion set,suction catheter
4. Why should you buy from us not from other suppliers?
With over 10years' experience in medical industry, we have strong sourcing capalibity and good connections with many factories for
medical apparatus and consumables. We always can provid best price, appropriate quality, excellent & professional service.
5. What services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW,CIP,FCA,CPT;
Accepted Payment Currency:USD;
Accepted Payment Type: T/T,L/C,Cash;
Language Spoken:English,Chinese
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





