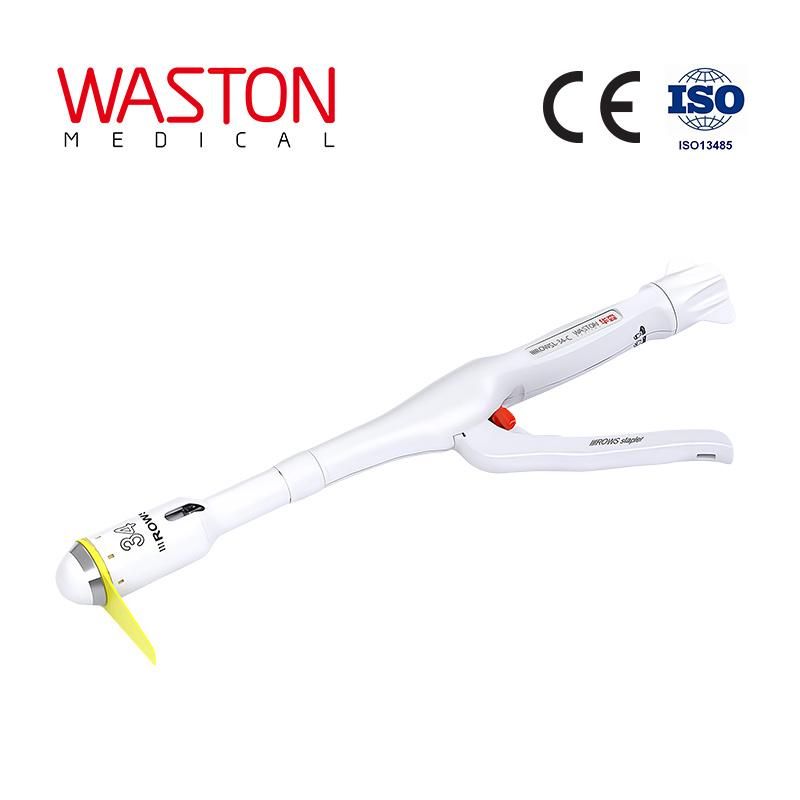
Surgical Stapler\3 Pph Stapler
Dong Guan Hong Xin Sporting Goods Co., Limited / 2022-06-23

- Material:ABS&Titanium&Ss
- Feature:Disposable
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:Male,Female
- Logo Printing:With Logo Printing
=== Base Info ===
- Certifications:CE ,ISO
- Transport Package:Non-Sterile Package, Paper Plastic Roll Bag
- Specification:CE, SGS
- Trademark:Waston Medical
- Origin:Changzhou, Jiangsu
- HS Code:9018909000
=== Description ===
Basic Info.
Certifications CE ,ISO Transport Package Non-Sterile Package, Paper Plastic Roll Bag Specification CE, SGS Trademark Waston Medical Origin Changzhou, Jiangsu HS Code 9018909000Product Description
Procedure for Prolapse and HemorrhoidsThe PPH Stapler is a uniquely designed set of instruments, developed to treatthe hemorrhoidal disease conservatively, repositioning the hemorrhoidal
cushions to their original location without excising them.
Features:·Single use, avoid the possibility of cross-infection.·Large cartridge housing capacity accommodate even extensive prolapses.·Controlled, stable and minimum dilatation of circular anal dilator.·Purse string suture anoscope provides simultaneous flattening of the prolapse
and supports introduction of the purse string suture.
 COMPANY INTRODUCTIONAs a national high-tech enterprise, the proportion of our R&D investment to total sales has been over 8% for consecutive years, realizing the transformation of the experts' clinical experience and their innovative ideas into achievements, launched several innovative products which have the national invention patents and patents for utility model. At present, we pioneer globally the revolutionary innovation technology of Rev Drill system,Three-Row-Stapler,Sternal Fixation and Rib plate, which are fully endorsed by both the experts and the market, as a national brand and benchmarking of innovative products in the domestic medical devices industry.
COMPANY INTRODUCTIONAs a national high-tech enterprise, the proportion of our R&D investment to total sales has been over 8% for consecutive years, realizing the transformation of the experts' clinical experience and their innovative ideas into achievements, launched several innovative products which have the national invention patents and patents for utility model. At present, we pioneer globally the revolutionary innovation technology of Rev Drill system,Three-Row-Stapler,Sternal Fixation and Rib plate, which are fully endorsed by both the experts and the market, as a national brand and benchmarking of innovative products in the domestic medical devices industry.
MANUFACTURE INTRODUCTION"Good faith as the base, quality above all" is Waston's Philosophy.
Waston operates strictly according to the ISO13485 quality system, at the same time proceed the specifications of medical devices manufacturing quality management, implants and sterile medical devices execution rules, as well as the CE MDD 93/42/EEC directive implementation.
In recent years we have formed the quality control system of international standard level by constant upgrading, standardization of management in details, improvement of the whole process control. We have also imported a lot of processing equipment, including the German-imported and American-imported machining centers, Japanese-imported automatic lathes and so on, ensuring our first-class quality by first-class equipment. Since 2011 we've invested over 20 million RMB in the expansion of our Testing Center, which is divided into a biomechanical laboratory and a physical-chemical testing center, providing powerful assurance to our three key critical points: raw material procurement control, product quality control and new product performance analysis.
 CONGRESS SHOWOur products are exported to many countries, such as Columbia, France, Turkey, Indonesia, Mexico, Russia, and Thailand etc. According to their feedbacks, the quality of our products can well meet the standards in their countries.
CONGRESS SHOWOur products are exported to many countries, such as Columbia, France, Turkey, Indonesia, Mexico, Russia, and Thailand etc. According to their feedbacks, the quality of our products can well meet the standards in their countries.

 O E MWaston is also as a major OEM supplier to some of the largest global players in this industry.
O E MWaston is also as a major OEM supplier to some of the largest global players in this industry. New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





