Sterilized Disposable Virus Specimen Collection Sampling Tube with Swab
Anping County Dinghe Wire Mesh Co., Ltd. / 2022-06-23

- Type:Virus Sampling Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:bd-01
- Transport Package:Box
- Specification:58*38*38
- Trademark:qinkai
- Origin:Heze
- Production Capacity:50000000
=== Description ===
Basic Info.
Model NO. bd-01 Transport Package Box Specification 58*38*38 Trademark qinkai Origin Heze Production Capacity 50000000Product Description
Product name: Viral tranport medium tube with swabModel / Type:20 tests / box, 40 tests / box, 50 tests / box, 100 tests / box
Intended use: The product is intended to be used for collection, transportation and storage of samples ( Viruses, chlamydiae, mycoplasma and ureaplasma).
Indication: Used for collection, storage and transportation of viruses, chlamydiae, mycoplasma and ureaplasma.
Contraindication: The product is strictly prohibited for the sampling of bacterial samples, and the preservative liquid itself contains antibiotics which inhibit the bacteria.
Test Population: Applicable for all population necessary for swabbing.
Intended users: Qualified doctors, nurses for specimen collection in a medical laboratory or hospital.
Component: It is usually composed of swabs and / or cups and tubes containning preservative liquid.
Scope of application: It is used for collection, transportation and storage of samples.
Precautions/cautions:
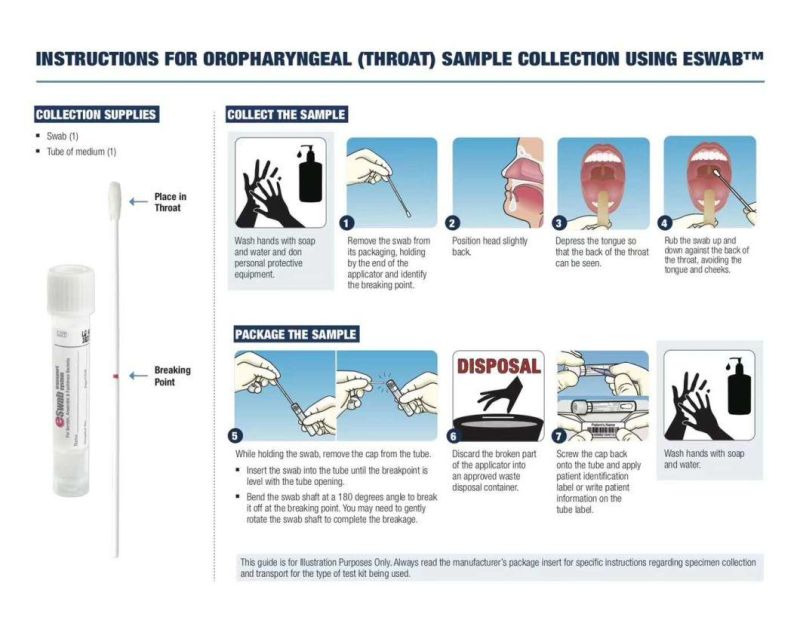
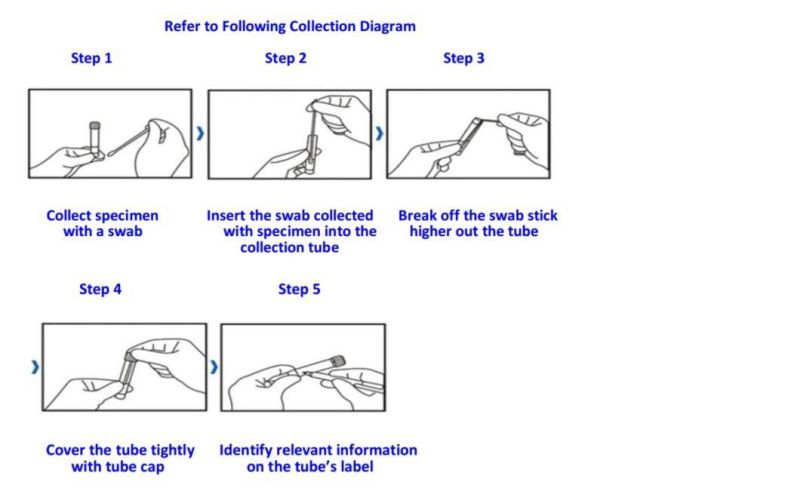
1. After sampling, the disposable sampler should be inspected as soon as possible, and transported immediately at low temperature of 2ºC-8ºC degrees. The samples collected can be stored for 2-8 hours, at a low temperature for 48 hours. The long term storage should be kept at low temperature below -20ºC, and stored at -70ºC or -196ºC.
2. The product is strictly prohibited for the sampling of becterial samples, and the preservative liquid itself contains antibiotics which inhibit the bacteria.
3. Avoid direct contact with patients being collected, and remove the wet swabs before sampling the patients.
4. Sampling should be done strictly according to the sampling procedure, so that the sampling location is accurate and the sampling intensity is uniform. Otherwise, the quality of sample collection will be affected.
5. The product should not be used after the expiration date or the product package is damaged.
Warning:
1. This product is a disposable product, so it is forbidden to reuse. After use, it should be treated as medical waste;
2. Before use, please pay attention to whether the packaging and the products are damaged or have other obviouis defects. Please check whether the product is within the validity period. If it is beyong the validity period, do not use.
3. Please read the relevant instructions carefully before use, and be familiar with the instructions and operation methods of the products.





Shandong Qinkai Medical Industry Co., Ltd.
Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





