Disposable Foam Applicator Antigen Test Swabs Specimen Collection Foam Swabs with CE0197
Ritscher Paper Products Limited / 2022-06-23

- Type:Specimen Collection
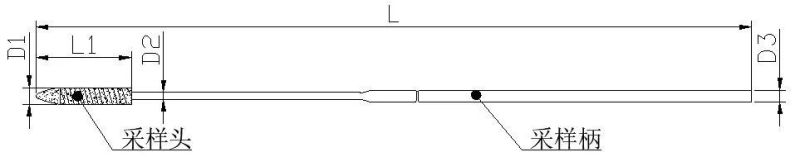
- Material:Polyurethane Sponge Tip+PP Shaft
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:5 Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
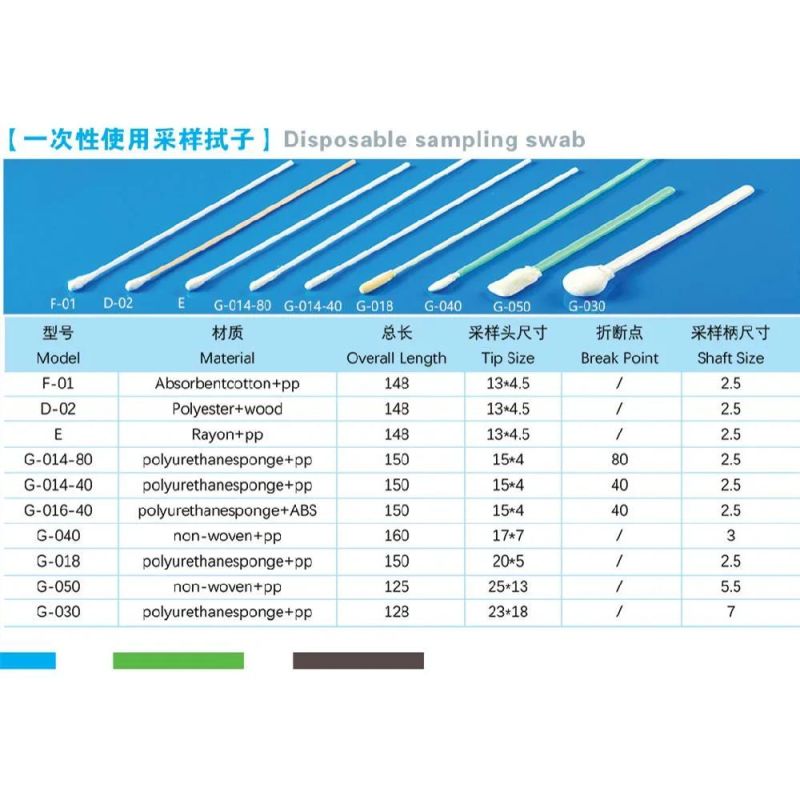
- Model NO.:G-090
- CE Registaration No.:Dd 60144541 0001
- FDA Owner Number:10061791
- ISO:13485
- Transport Package:Soft Blister, 100PCS ,Zip Bag, 100 Bags,Carton
- Specification:60*42*40
- Trademark:HANHENG MEDICAL
- Origin:China
- HS Code:3005901000
- Production Capacity:20000000PCS,Week
=== Description ===
Basic Info.
Model NO. G-090 CE Registaration No. Dd 60144541 0001 FDA Owner Number 10061791 ISO 13485 Transport Package Soft Blister, 100PCS /Zip Bag, 100 Bags/Carton Specification 60*42*40 Trademark HANHENG MEDICAL Origin China HS Code 3005901000 Production Capacity 20000000PCS/WeekProduct Description
| Model | Desciption | Packing | Product Application | Break point |
| G-090 | Polyurethane sponge tip+ PP shaft , EO Sterile | 1pc Individual packing, 100pcs/zip bag; 100 bags/carton, 10000PCS/Carton Carton Size: 60*42*40 12KG/G.W. 11KG/N.W. |
| Can be customized |
- Diagnostic Cell Collection, High Particle Collection Capacity, DNA Testing.
- Medical-grade polyurethane foam has been found to be an excellent tip material for diagnostic.
- Soft, small tip for patient comfort.
- Lint and residue free.
- Adhere to guideline of CDC.
- Improve diagnostic sensitivity.
- Great liquid absorbent of liquid specimen &rapid elution to media.
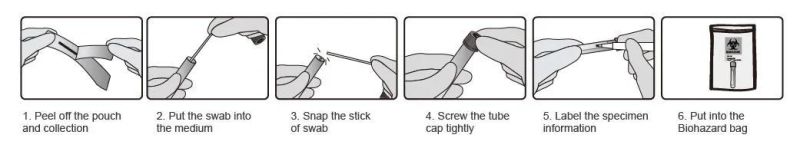
- Easy handling and transport.
- No background from RNAse or other contaminants for test accuracy.







New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





