Hot Sale Medical Disposable Infusion Set with Luer Lock, Disposable IV Set with Low Price
Anping County Dinghe Wire Mesh Co., Ltd. / 2022-06-23
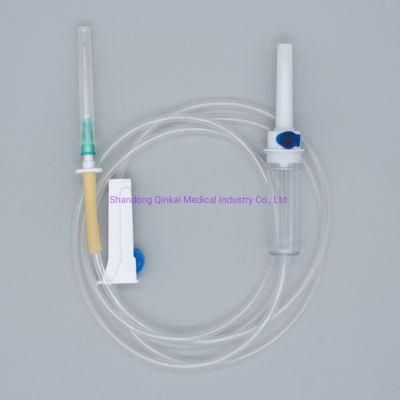
- Type:Infusion Set
- Material:PVC
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:OEM
=== Base Info ===
- Model NO.:QK-06
- Transport Package:PE
- Specification:61*38*32.5CM
- Trademark:Qinkai
- Origin:China
- HS Code:9018390000
- Production Capacity:2000000pieces,One Day
=== Description ===
Basic Info.
Model NO. QK-06 Transport Package PE Specification 61*38*32.5CM Trademark Qinkai Origin China HS Code 9018390000 Production Capacity 2000000pieces/One DayProduct Description
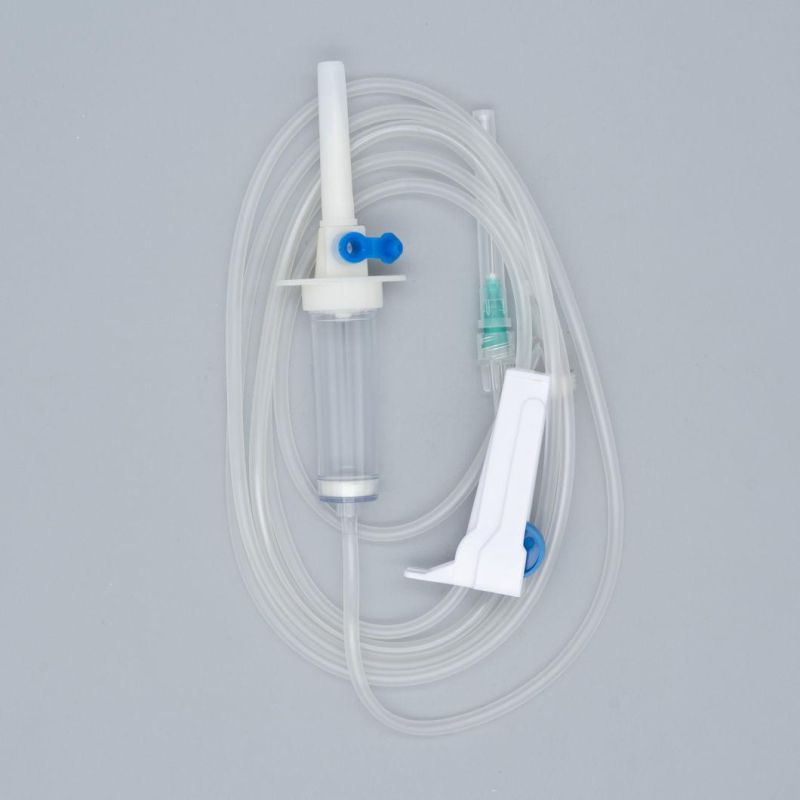 Specifications
SpecificationsDisposable Infusion Set
CE ISO approved
For Eur,South America,Midest and Africa markets
Factory directly price
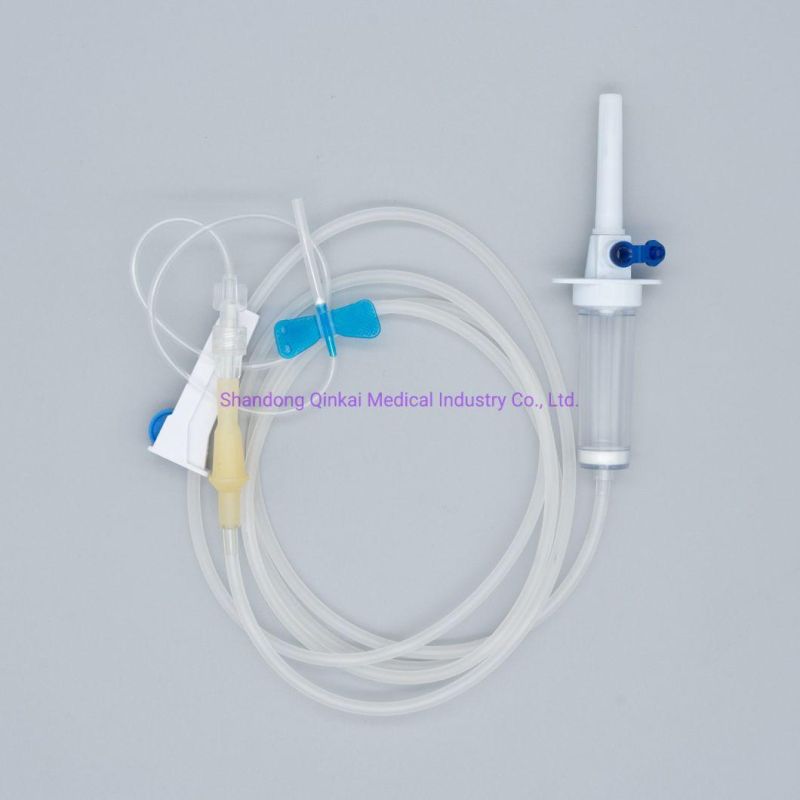
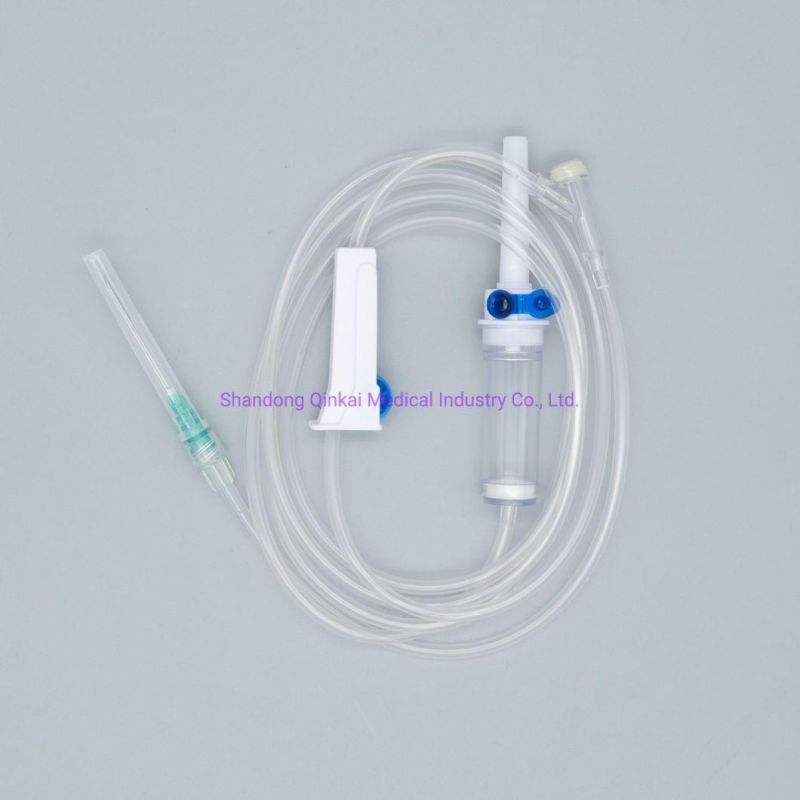
Disposable IV infusion set/Pediatric infusion set
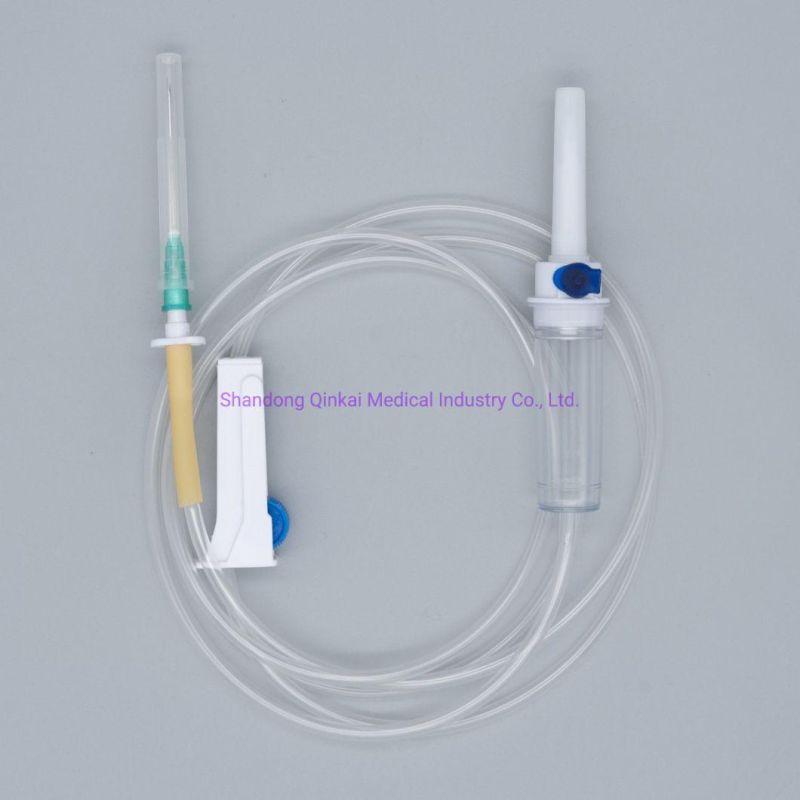
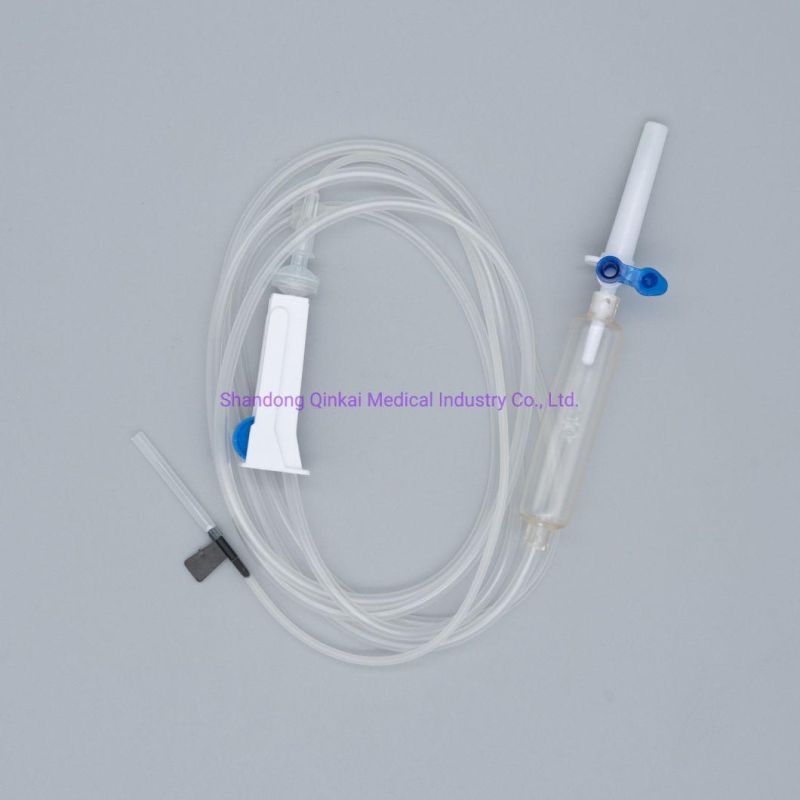
Main accessories: Vented spike, Drip Chamber, fluid Filter, flow regulator, latex tube, Luer Lock connector.
Protective cap for closure piercing device made of polyethylene with internal thread that prevents the bacteria from coming in, but allows the
entrance of ETO gas. Apporoximately 15 drops/ml,20 drops/ml
Closure piercing device made of white PVC, with sizes according to ISO 1135-4 standards. Apporoximately 15 drops/ml,20 drops/ml
Drip chamber made of soft PVC, sizes according to the ISO 8536-4 standards.
Flow Regulator made of polyethylene.
Soft and kink resistant medical grade PVC tubing .
Terminal fitting protective cap (luer slip or Luer-lock adapter upon request) made of PVC or polystyrene, according to the ISO 594/1 and 594/2
standards.
Terminal fitting protective cap made of polyethylene.
Options available :
- With or without air vented spike;
- With or without needle;
- With or without "Y" injection port;
- Luer lock or luer slip connector;
Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





