Medical Supply Nonwoven Absorbent Disposable Ent Split Surgical Drape
Shandong Yusheng Biotechnology Co., Ltd. / 2022-06-23
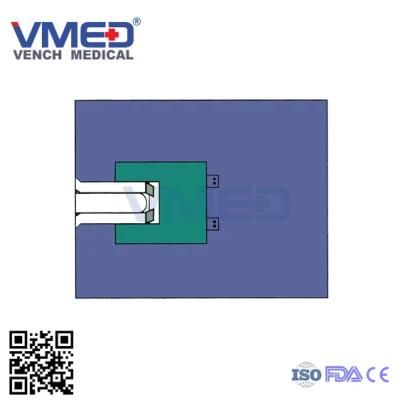
- Type:Surgical Supplies Materials
- Material:SMS+PP
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:Without Logo Printing
=== Base Info ===
- Model NO.:VESD
- Transport Package:Carton
- Specification:100*130cm,150*250cm,220*300cm Various size will be
- Trademark:OEM,VMED
- Origin:China
- HS Code:3005901000
- Production Capacity:5000000 Piece,Month
=== Description ===
China
Other products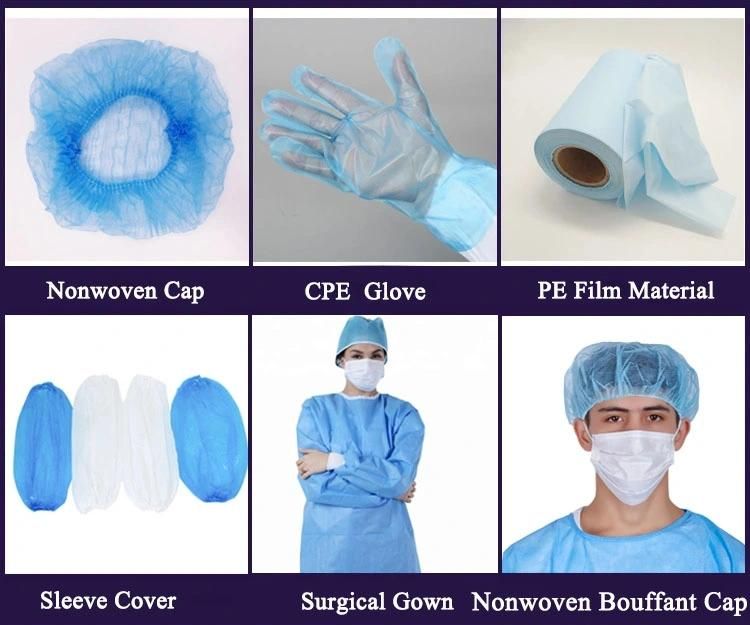

Material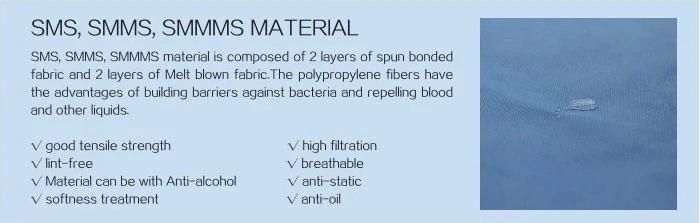


Factory
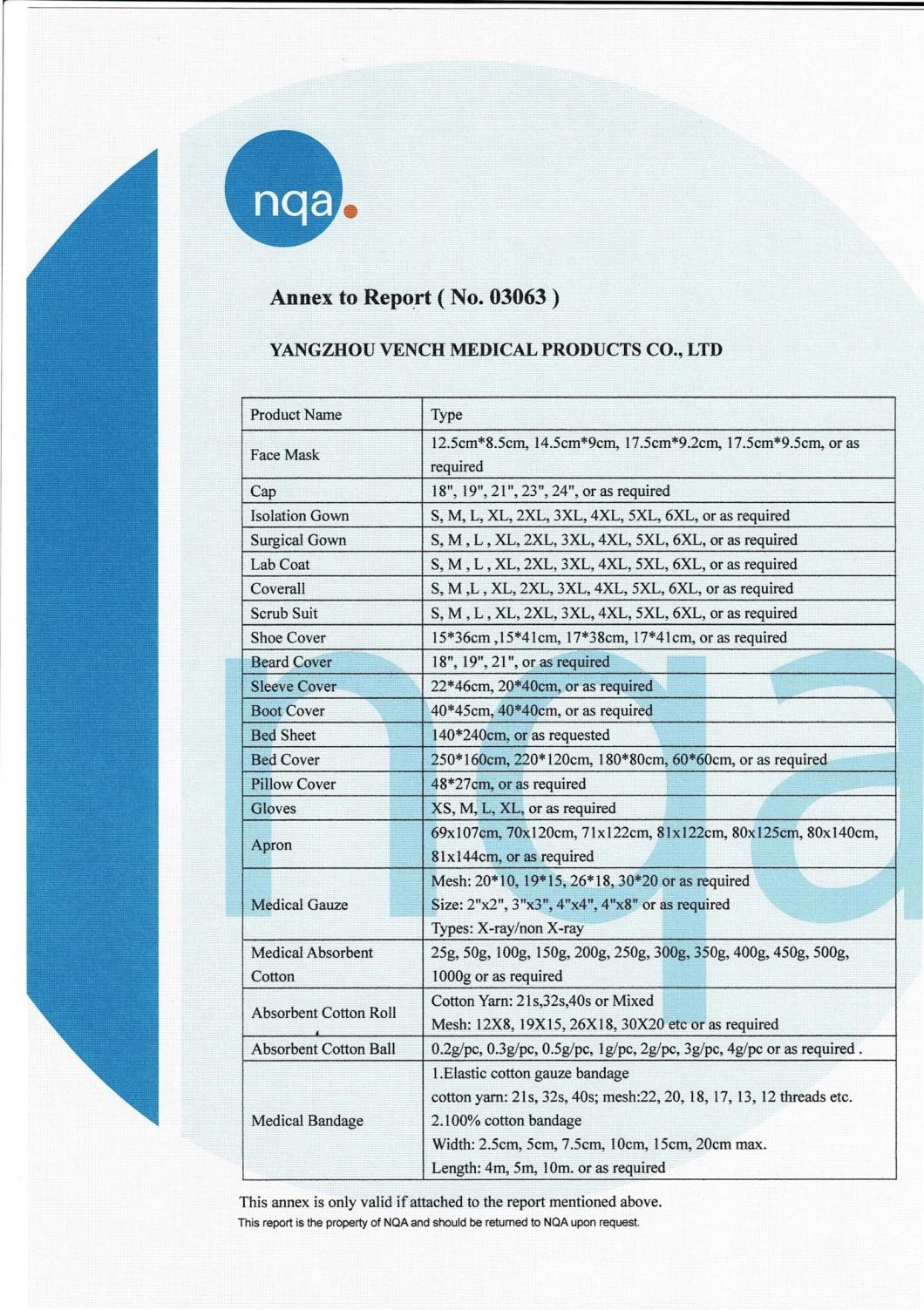
Certifications



FAQ
Q1: Can I have a sample first ?
VMED:Of course ! Sample are available and free
Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)?
VMED: Yes, we have CE, ISO13485, FDA,
Q3:What's your advantage? Why we choose you?
VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products.
Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
Q4 : How does your factory control the quality?
VMED : All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
Q5: What the expiration date for disposable non-woven products?
VMED: We suggest 2 years of shelf storage
Trust us
-- professional &safety
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





