FDA CE Approved DNA Rna Test Kit Inactivated Inactivation Nasal Transport Medium Vtm Disposable Specimen Collection Virus Sampling Tube
Shenzhen Oskyoo Technology Co., Limited / 2022-06-23
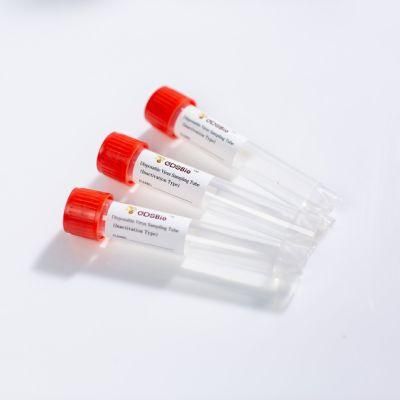
- Type:Sampling Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:One Year
- Group:All People
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:ST04
- Transport Package:Carton Packaging
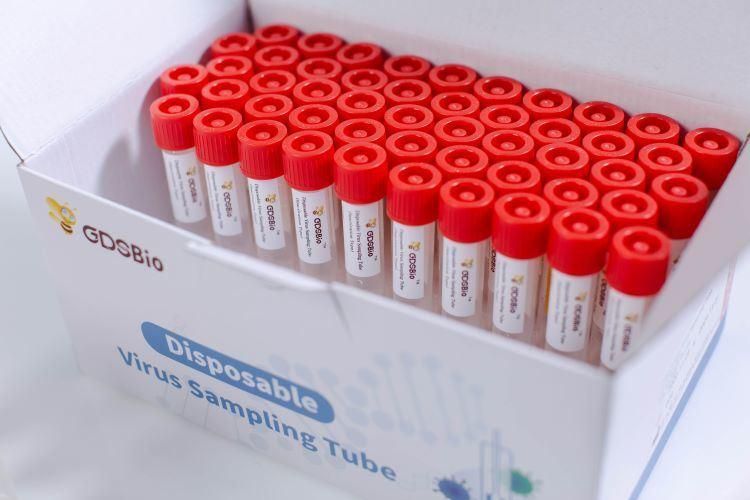
- Specification:50 pieces,box
- Trademark:GDSBio
- Origin:China
- HS Code:3822001000
- Production Capacity:500000,Week
=== Description ===
Basic Info.
Model NO. ST04 Transport Package Carton Packaging Specification 50 pieces/box Trademark GDSBio Origin China HS Code 3822001000 Production Capacity 500000/WeekProduct Description
GDSBio provides various types of sampling tubes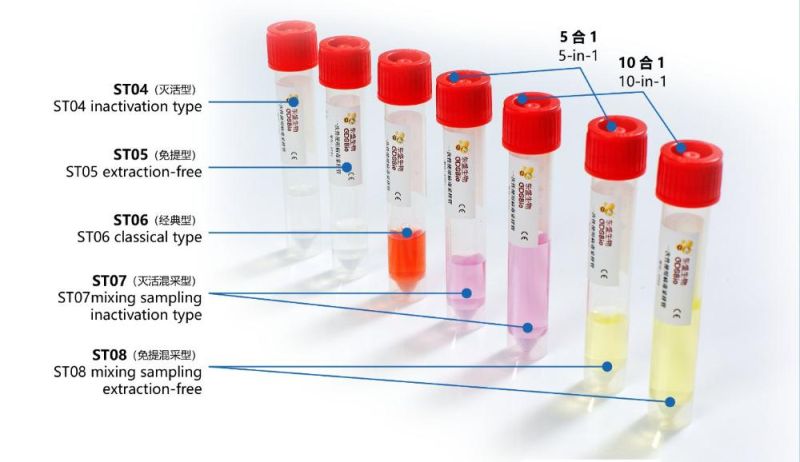 Product Name
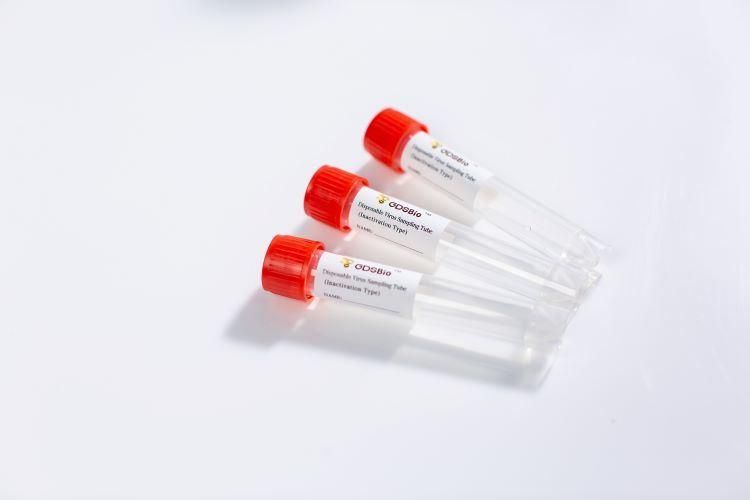
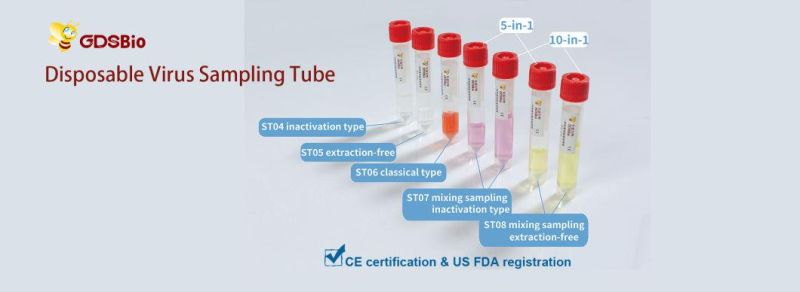
Product NameDisposable Virus Sampling Tube (Inactivation Type)
Packing Components
| Cat. No. | Specification of Tube | Volume of Preservation Solution | Package Size |
| F4001a | 2 ml | 1 ml | 1 pc/bag; 50 pcs/box |
| F4002a | 5 ml | 2 ml | 1 pc/bag; 50 pcs/box |
| F4003a | 10 ml | 3 ml | 1 pc/bag; 50 pcs/box |
| Cat. No. | Specification of Tube | Volume of Preservation Solution | Package Size |
| F4001b | 2 ml | 1 ml | 1 set/bag; 50 sets/box |
| F4002b | 5 ml | 2 ml | 1 set/bag; 50 sets/box |
| F4003b | 10 ml | 3 ml | 1 set/bag; 50 sets/box |
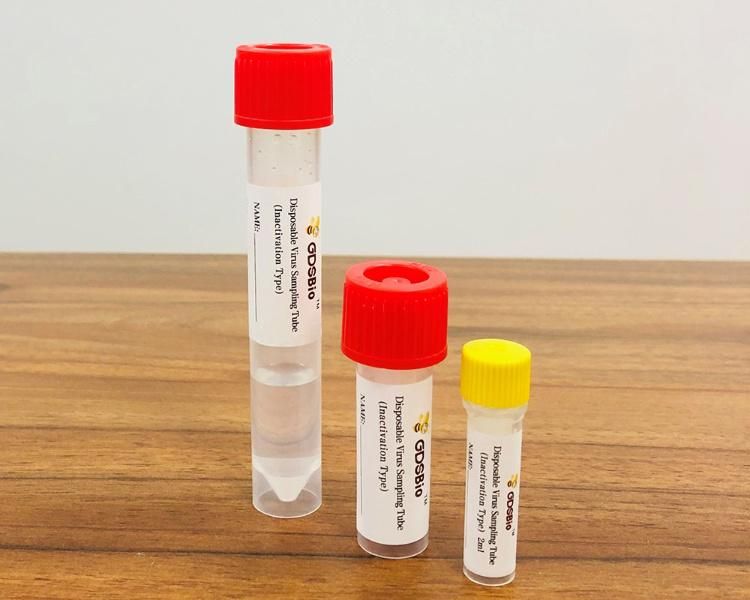 Intended Use
Intended UseDisposable Virus Sampling Tube (Inactivation Type) is used for the collection, transportation and storage of virus specimens.
Storage Conditions and Shelf Life
Stored at 5 ~ 25°C, with a validity period of 12 months.
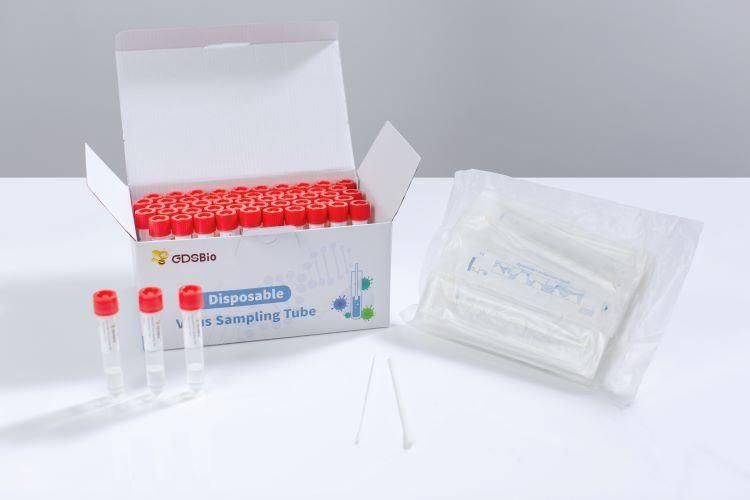
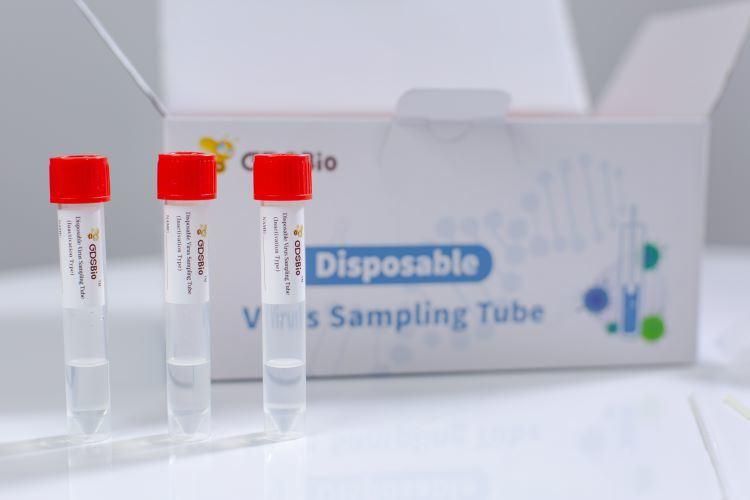
 Patented formula, contains highly efficient lysate for rapid lysis and inactivation of virus, and eliminate biosafety hazards
Patented formula, contains highly efficient lysate for rapid lysis and inactivation of virus, and eliminate biosafety hazardsSafe/High Nucleic Acid Yield/Room Temperature Stable
Certification: CE/ US FDA/ China FDA
Certificate for Exportation

Nylon-flocked ABS stick nasal swab, throat/oral swab
 Environmentally friendly and strong carton packing
Environmentally friendly and strong carton packing
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





