Eo Sterile Non Woven Surgical Knee Arthroscopy Drape Suppliers with CE ISO13485 Certification
Lanxi Kingway International Trade Co., Ltd. / 2022-06-23
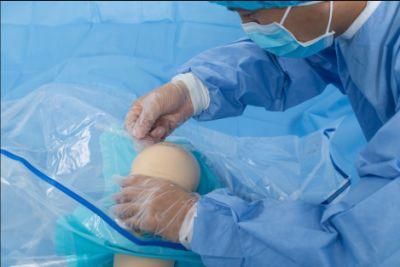
- Material:PP
- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:Knee Patient
=== Base Info ===
- Model NO.:200*300cm
- Logo Printing:Without Logo Printing
- Transport Package:Individual Packing, 50PCS,CTN
- Specification:SMMS,SPP
- Trademark:JOINKONA, OEM
- Origin:China
- HS Code:6307900090
- Production Capacity:50000PCS,Day
=== Description ===
Open the package; use it according to the medical routine
Antimicrobial Incise Drapes provide a unique barrier to bacterial contamination and its advanced antimicrobial properties reduce the growth and migration of skin flora during surgical procedures, reducing the risk of surgical site infection
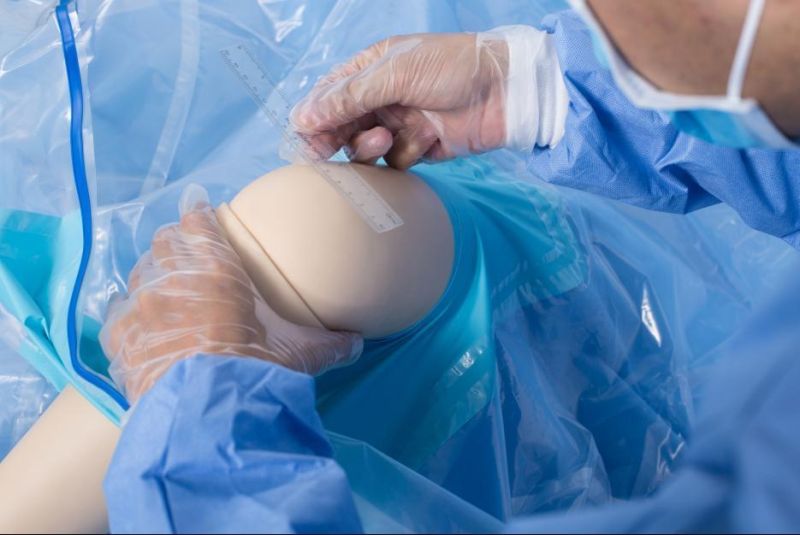
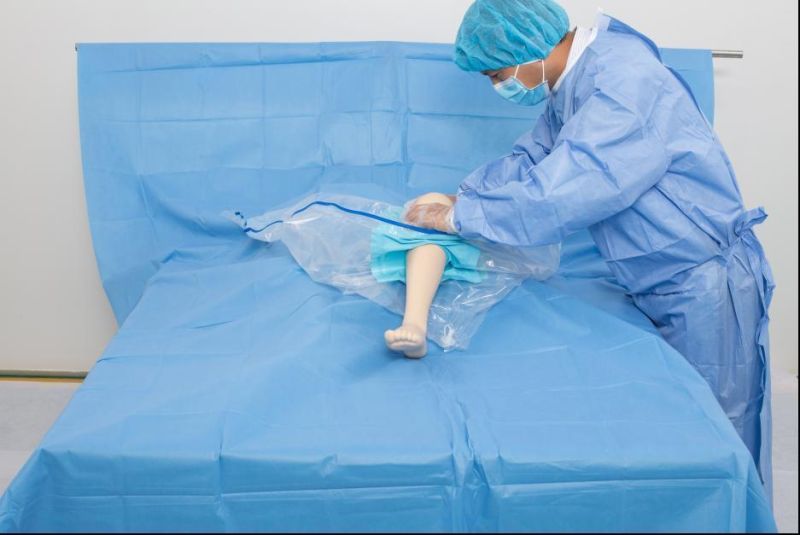
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





