Pinmed Medical Disposable Insulin Syringe 0.3ml/0.5ml/1.0ml CE&ISO
Suzhou Orange Technology Limited Company / 2022-06-23
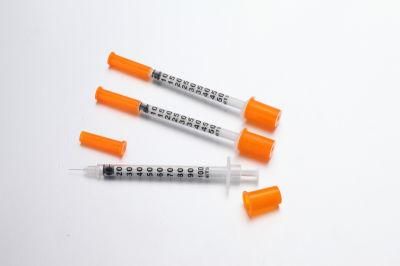
- Material:PVC
- Feature:Disposable
- Certification:CE, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:Male
=== Base Info ===
- Model NO.:PM10012
- Logo Printing:With Logo Printing
- Validity:5 Year
- Transport Package:Blister Bag
- Specification:0.5,1ml
- Trademark:PINMED
- Origin:China
- HS Code:9018310000
- Production Capacity:1000000000PCS,Month
=== Description ===
Basic Info.
Model NO. PM10012 Logo Printing With Logo Printing Validity 5 Year Transport Package Blister Bag Specification 0.5/1ml Trademark PINMED Origin China HS Code 9018310000 Production Capacity 1000000000PCS/MonthProduct Description
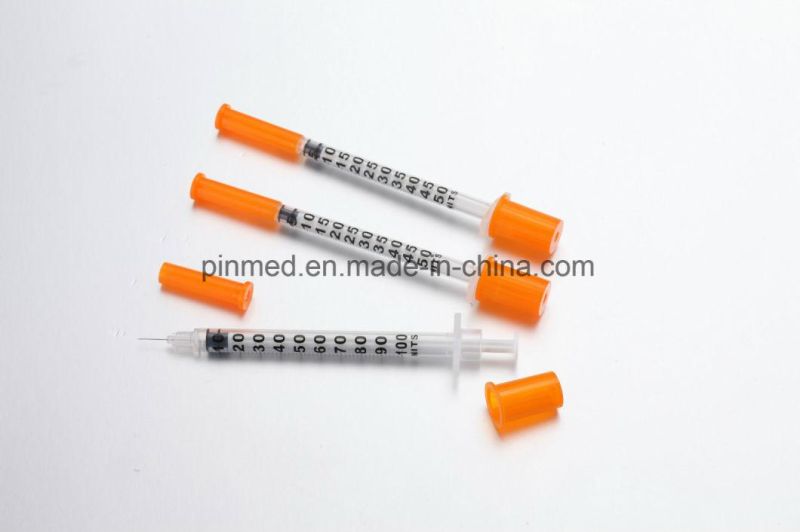
1)Sterilized by EO gas, non-toxic, non-pyrogenic, single use only.
2) Ultra fine cannula for maximum patient comfort
3) Product structure: barrel, plunger, piston, and sterile hypodermic needle
4) Insulin syringe size of 1ml , 0.5ml are available 100units or 40units
5) made by medical polymer materials, EO sterilized
6) The orange color of the cap helps the patient easily discriminate the insulin
Packed in individual blister bag
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





