Home Use Self-Testing Coid-19 Antigen Rapid Test Cassette for EU Market From Original Manufacturer Uni-Medica with CE
Tsingtao Skyline International Trading Co., Ltd. / 2022-06-23
- Type:Test Strips & Test Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:One Year
- Group:All People
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:R041-01
- Transport Package:Standard Export Carton
- Specification:20 tests,kit
- Trademark:uni-medica
- Origin:China
- HS Code:300215
- Production Capacity:100 000 000 Tests,Year
=== Description ===
Basic Info.
Model NO. R041-01 Transport Package Standard Export Carton Specification 20 tests/kit Trademark uni-medica Origin China HS Code 300215 Production Capacity 100 000 000 Tests/YearProduct Description
Product DescriptionThis Antigen Test Kit is used for in vitro qualitative detection of virus antigens in human throat swabs and nasal swabs.
This Antigen Test Kit is limited to medical institutions.
The virus belongs to the β genus. New virus pneumonia is an acute respiratory infectious disease, and the population is generally susceptible. The main source of infection is the patients who has been infected by the virus, and the asymptomatic carrier may also be the source of infection. Based on the current epidemiological investigation, the incubation period is 1-14 days, mostly 3-7 days. The main manifestations are fever, dry cough, and fatigue. A small number of patients are accompanied by nasal congestion, runny nose, sore throat, myalgia and diarrhea.
Testing Principle
The virus Antigen Kit(colloidal gold) uses immunochromatography technology and adopts the principle of double antibody sandwich method for detection. During the chromatography, the virus antigen in the sample reacts with the colloidal gold-labeled virus antibody 1 to form a complex, which moves forward along the nitrocellulose membrane under the action of chromatography. In the detection area, it combines with the virus antibody 2 coated on the nitrocellulose membrane to form a sandwich complex and deposits on the detection line (T line) to form a purple-red band; on the contrary, if the sample does not contain the virus antigen, the purple-red band will not form on the detection line (T line). Regardless of whether the sample contains the virus antigen, the chicken IgY in the gold label pad and the goat anti-chicken IgY on the detection line (C line) will form a specific binding, which forms a purple-red band at the position of the detection line (C line).
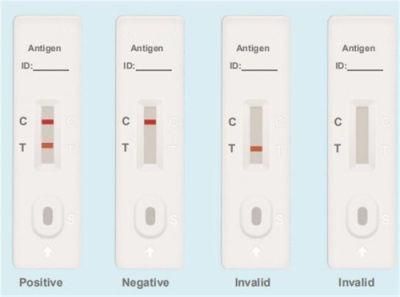
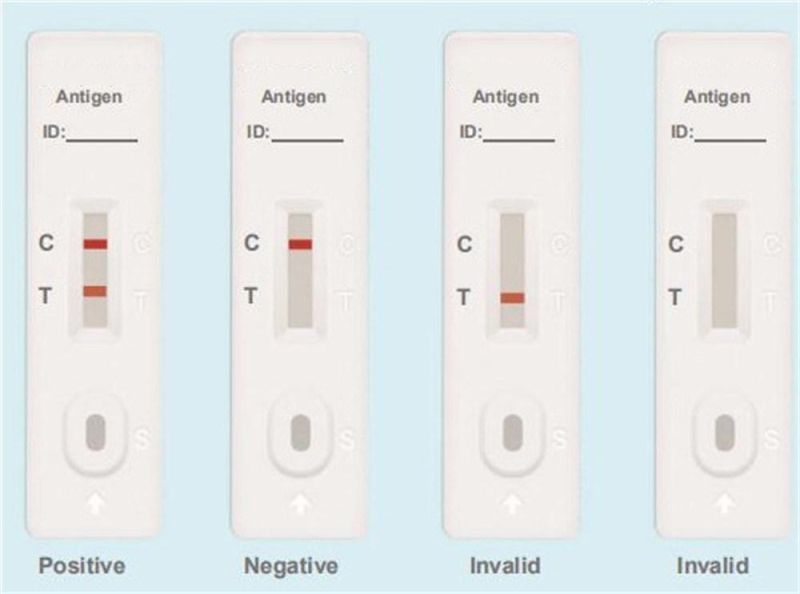
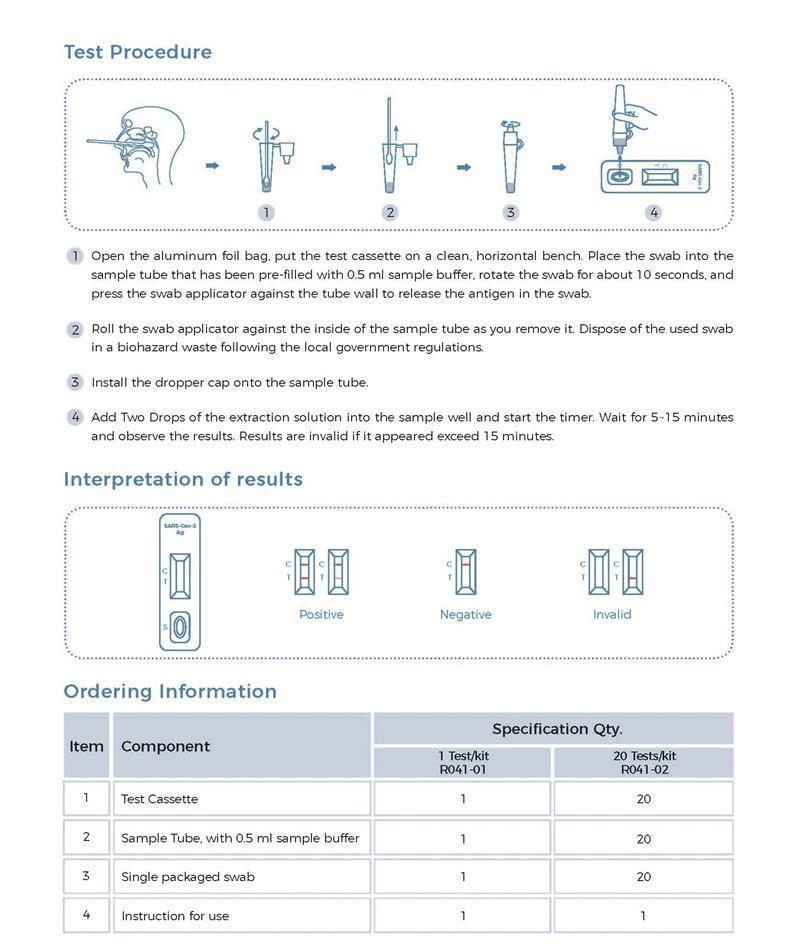
Testing Results
Positive: Both the detection line (T line) and the quality control line (C line) appear colors.
Negative: The test line (T line) does not appear color, only the quality control line (C line) appears color.
Invalid: The quality control line (C line) does not appear color, or both of the quality control line (C line) and the test line (T line) do not appear color, which means that the test is invalid and the test should be repeated.
 Scope of Use
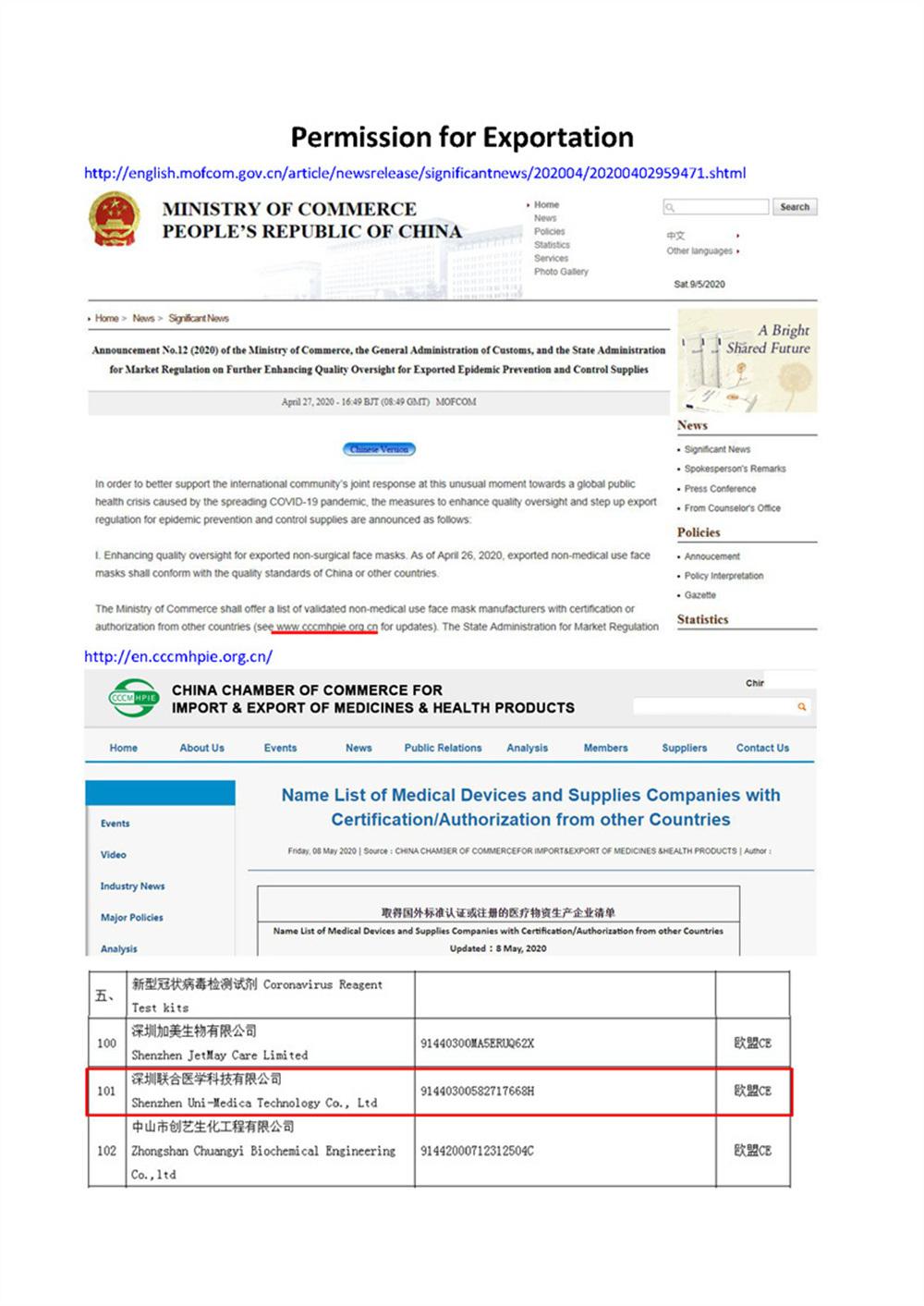
Scope of UseEU countries and countries that recognize the EU CE certification.
Product Features
1. Specimen: Nasal / Throat l Anterior Nasal swab
2. Simple operation
3. Convenient storage: 2-30°c
4. High sensitivity and specificity
Our advantage
1. Quick result: 10 minunites
2. High accuracy: >99%
3. High sensitivity:>99%




New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





