CE&ISO Approved IV Catheter, Injection Port, Pen-Like, with Wings
Nanjing Neg Optoelectronics Co., Ltd. / 2022-06-23

- Type:Infusion Set
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:5 Years
- Group:Adult
- Logo Printing:with,Without Logo
=== Base Info ===
- Model NO.:Injection Port, Pen-Like, with Wings
- OEM:Accept
- Transport Package:Blsiter Packing, Middle Box, CTN
- Specification:14G-26G
- Trademark:TrusCare Medical
- Origin:China
- HS Code:9018390000
- Production Capacity:500000 PCS,Month
=== Description ===
Basic Info.
Model NO. Injection Port, Pen-Like, with Wings OEM Accept Transport Package Blsiter Packing/ Middle Box/ CTN Specification 14G-26G Trademark TrusCare Medical Origin China HS Code 9018390000 Production Capacity 500000 PCS/MonthProduct Description
Disposable I.V. Catheter/ I.V. CannulaProfile:
CE and ISO certificate
High quality and reasonable price
Various types
Usage:
Used for clinical peripheral vascular system insertion, repeated infusion / blood transfusion, parenteral nutrition, emergency rescue, etc
Main Raw Material :
FEP
ABS
SUS304


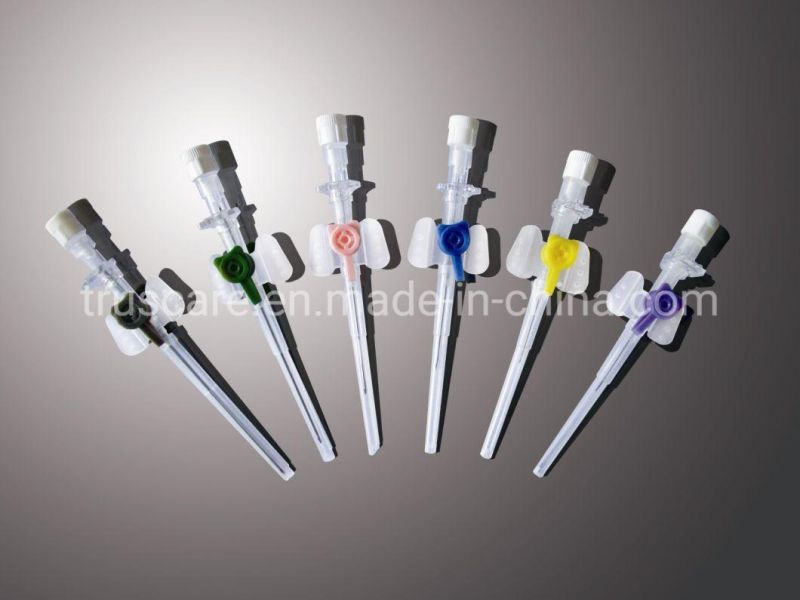
Type:
IV Catheter Pen-Like Model
IV Catheter with Wings
IV Catheter with Injection Port
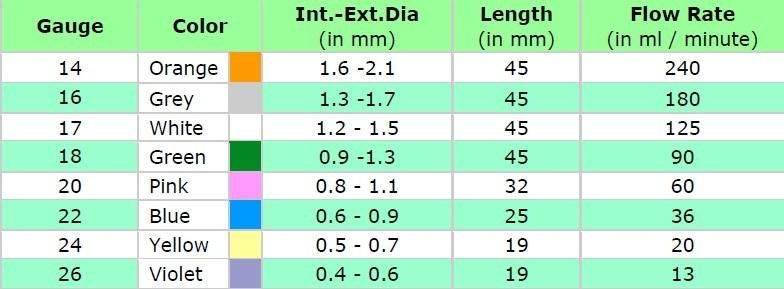
Specification:
- Advanced tip design, made of kink resistant and biocompatible FEP, with extremely smooth inner and outer surface to ensure easy vein puncture with minimum trauma.
- Color-coded easing cap allows for easier identification of catheter size.
- Gently tapered catheter tip aligns with the needle bevel to provide smooth transition from needle to catheter.
- Catheter with 3 stripes embedded X-ray contrast lines.
- Siliconized and ground stainless steel needle with ultra-sharp triple facet bevel for smooth and painless vein puncture.
- Use of Hydrophobic membrane filter eliminated blood leakage.
- Sterilized by EO gas, non-toxic, non-pyrogenic
- Size from 14 G TO 26G
- Shelf time: three years
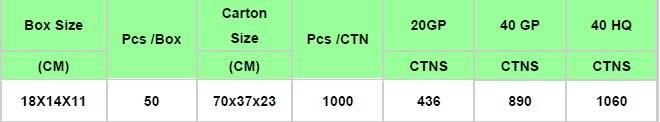
Package:
Individually packed by blister packing, secondary packed by box, outer packed by carton.
Precaution:
- Don't use if the single package damaged or protective cover falling off or there's foreign matter internally.
- Do not attempt to reinsert a partially or completely withdrawn needle.
- The product is not allowed remaining in vein over 72 hours.
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





