Cuffed Uncuffed Reinforced Disposable Medical Endotracheal Tube with CE FDA Certificate
ZHENGZHOU KING PACK LONG MACHINERY CO., LTD. / 2022-06-23
- Type:Catheter
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:All Persons
- Logo Printing:With Logo Printing
=== Base Info ===
- Biocompatible:Yes
- Sterile:Eo
- Quality:Excellent
- Price:Competitive
- Delivery Time:Fast
- Transport Package:Packed in Carton, Shipped by Sea, Air, Express
- Trademark:OEM, ODM
- Origin:China
- HS Code:9018390000
- Production Capacity:1000000000000PCS,Year
=== Description ===
Basic Info.
Biocompatible Yes Sterile Eo Quality Excellent Price Competitive Delivery Time Fast Transport Package Packed in Carton, Shipped by Sea, Air, Express Trademark OEM, ODM Origin China HS Code 9018390000 Production Capacity 1000000000000PCS/YearProduct Description
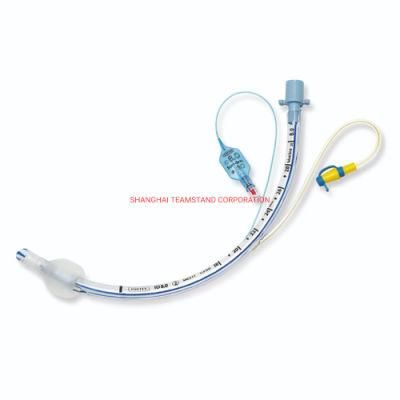
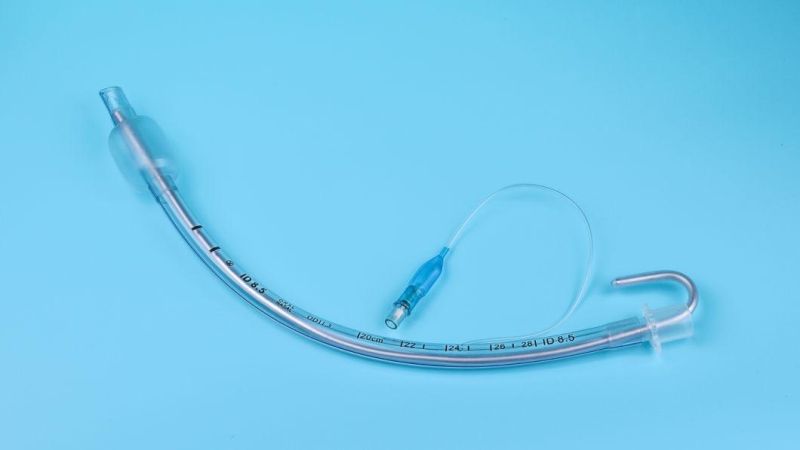
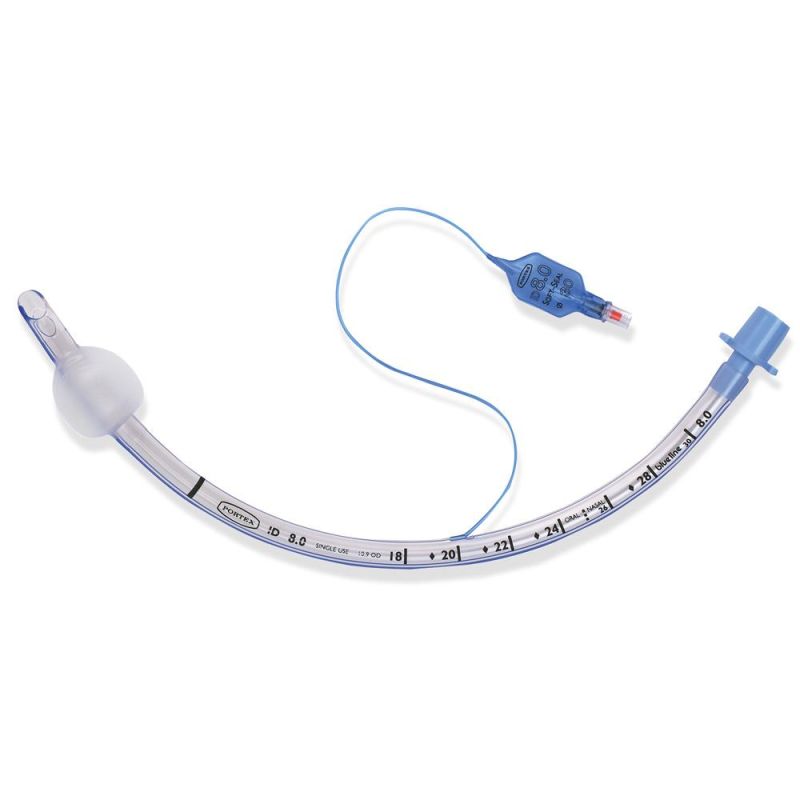
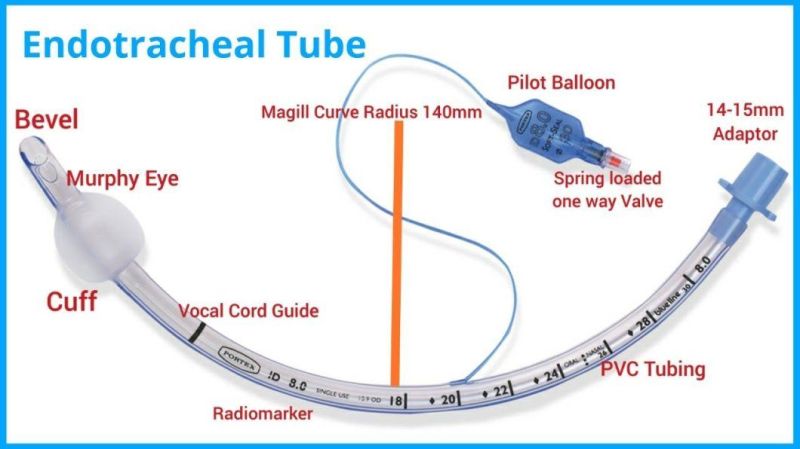
Endotracheal Tubes for single use are made of medical grade biocompatible materials (refer to BOM).The Endotracheal Tubes is a hollow cylinder inserted orally or nasally into the trachea to provide an unobstructed airway to convey gases and vapours to and from the lungs during anaesthesia, resuscitation, and other situations where the patient is not properly ventilated. The device may: 1) be packaged with a connector that will attach to a breathing circuit or manual resuscitator; 2) have a distal inflatable cuff to seal against the tracheal wall; 3) be radiopaque; and 4) have a built-in pilot balloon for cuff pressure monitoring. It is typically made of plastic or rubber and is available in various diameters and lengths for adult and paediatric patients.
Cuffed devices are designed to seal and protect the trachea from aspiration and to provide an unobstructed airway in patients during spontaneous, assisted or controlled ventilation for short or prolonged durations.
Product is delivered sterile. Sterilization process is validated according to EN ISO 11135. Sterilization process undergoes routine control.
The devices are single use.
The Endotracheal tubes is intended to insertion through the larynx into the trachea to convey gases and vapours to and from the trachea.









New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





