CE Approved Bladed Trocar for Gynecology Surgery
Hebei Zhouwo Trading Co., Ltd. / 2022-06-23
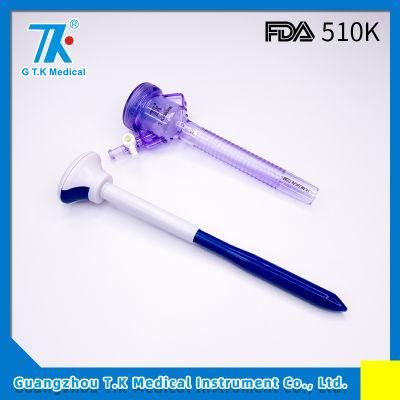
- Type:Medical Devices
- Material:Metal, Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Three Years
- Group:Laparoscopic Surgery Patients
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:T10-100-CT-A
- Transport Package:Blister Box Packaging
- Trademark:TK
- Origin:China
- HS Code:9018909000
- Production Capacity:100000
=== Description ===
Overview
Company ProfileProduct DescriptionDetailed Photos
Product Parameters Certifications

FAQ

| Q: Are you a factory or trading company? |
| A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience. |
| Q: What is the core strength of GTK Medical? |
| A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products. |
| Q: What makes GTK Trocars stand out? |
| A: Excellent sealing performance plus excellent smooth insertion and removal of surgical instruments. |
| Q: Do you provide free samples of GTK Trocars for clinical trial use? |
| A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation. |
| Q: Do you have exported experiences to large medical devices company/companies? |
| A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe. |
| Q: Do you accept factory audit prior to formal partnership? |
| A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021. |
Please do not hesitate to contact me to get best quotation!
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





