FDA 510k Antibacterial/ Antimicrobial Silver Foam Dressing for Wound Care
Guangdong Fowa Holdings Co., Ltd. / 2022-06-23

- Material:Foam
- Feature:Disposable
- Certification:FDA, ISO13485
- Ethylene Oxide Sterilization:Radiation
- Application:Hospital
- Group:All
=== Base Info ===
- Model NO.:LW-D-002
- Logo Printing:Both Ok
- FDA 510k:Silver,Antibacterial
- Silver Ion:Soft,High Exudate Absorbency
- Silver Monomer:Different Size Available
- Different Shapes:Antibacterial in 7 Days
- Transport Package:10PCS,Aluminum Paper Bag, One Bag One Box
- Specification:FDA 510K
- Trademark:LUOFUCON
- Origin:China Huizhou
- HS Code:3005901000
- Production Capacity:10, 000, 000PCS,Year
=== Description ===
Basic Info.
Model NO. LW-D-002 Logo Printing Both Ok FDA 510k Silver,Antibacterial Silver Ion Soft,High Exudate Absorbency Silver Monomer Different Size Available Different Shapes Antibacterial in 7 Days Transport Package 10PCS/Aluminum Paper Bag, One Bag One Box Specification FDA 510K Trademark LUOFUCON Origin China Huizhou HS Code 3005901000 Production Capacity 10, 000, 000PCS/YearProduct Description
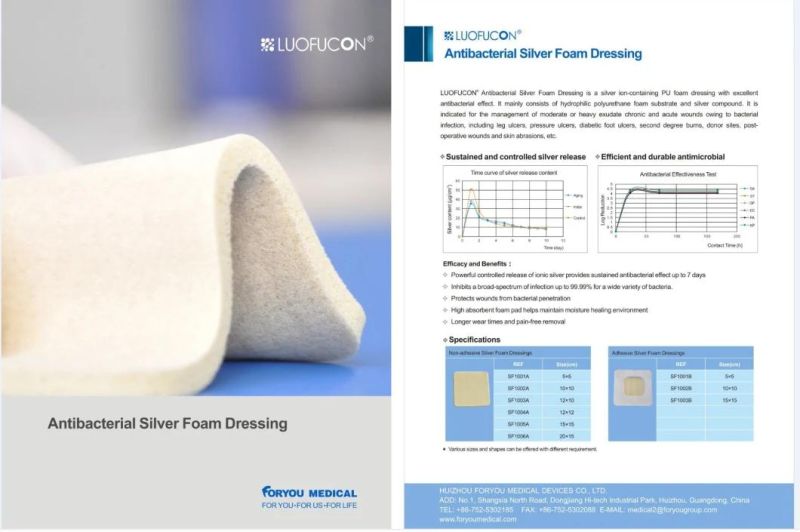

LUOFUCON Silver PU Antibacterial Foam Dressing is a highly absorbent foam dressing with ionic silver. The dressing is suitable for exuding, deep wounds and is perforated to ensure flexible handling. Diffrent size available.
Indications
Moderately to heavily exuding partial to full thickness wounds, including:
1. Trauma wounds
2. Pressure ulcers
3. Diabetic ulcers
4. Leg ulcers
5. Graft and donor sites
6. First-degree and second-degree burns
7. Post-operative surgical wounds
Contraindications
1. Do not useLUOFUCON® Silver PU Antibacterial Foam Dressing on patients with known sensitivity to silver.
2. LUOFUCON® Silver PU Antibacterial Foam Dressing is not intended for use on third-degree burns.
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





