Non Compliant High Pressure Balloon Catheters Nc Balloon Dilatation Catheter
Cangzhou Yunfeng Packaging Products Co., Ltd. / 2022-06-23
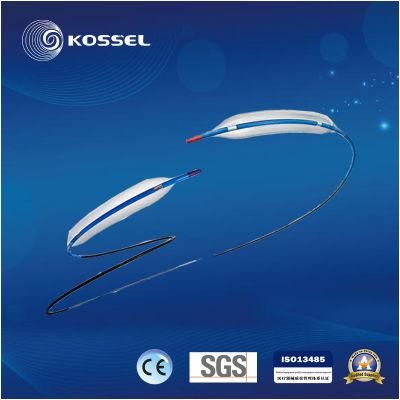
- Type:Catheter
- Material:Nylon
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Three Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
- Application:Cardiovascular
- Certification:ISO13485
- Nominal Burst Pressure:12 ATM
- Rated Burst Pressure:22ATM
- Balloon Folding:3
- Catheter Lengthcatheter Length:142cm
- Transport Package:Box or Pouch
- Specification:Diameter from 1.75mm to 5.0mm
- Trademark:OEM, Global
- Origin:Suzhou China
- HS Code:9018390000
- Production Capacity:5000PCS,Month
=== Description ===
Basic Info.
Application Cardiovascular Certification ISO13485 Nominal Burst Pressure 12 ATM Rated Burst Pressure 22ATM Balloon Folding 3 Catheter Lengthcatheter Length 142cm Transport Package Box or Pouch Specification Diameter from 1.75mm to 5.0mm Trademark OEM, Global Origin Suzhou China HS Code 9018390000 Production Capacity 5000PCS/MonthProduct Description

Product Features:
1. Material has excellent resistance to high pressure, NP-12atm and RBP-22atm, to provide good dilation performance in calcified lesions
2. Low compliance design and low inflation rate from NP to RBP supply accurate concentrations of expansion size and optimizing expansion effect
3. A short taper shoulder reduces the excess expansion at balloon ends, allows for a better focus on the target lesion and minimizes the damages to the vessel
4. A super slippery hydrophilic coating improves the catheter's ability to push forward and decreases operation time
5. Brilliant performance of folding after balloon expansion allowing for easier withdrawal
Size Matrix
| L:mm D:mm | 6 | 8 | 9 | 10 | 12(NP) | 14 | 15 | 16 | 18 | 20 | 22(RBP) | 24 | 25 | 26 | 27 | 30 |
| 1.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
Production Line
Kossel Medtech (Suzhou) Co., Ltd was founded in 2013, specializing in coronary,peripheral intervention medical devices, electrophysiology series products and other related accessories. We also welcome OEM/ODM cooperation.
Our initial team members are experienced field experts from China and The USA. Our current core teams have more than 10 years of experience in the field and our total staff number exceeds 200 people.
With consistent innovation abilities, advanced production capacity and 7,000 square meters of professional facilities and laboratories; we have obtained more than 100 intellectual property rights or patents, passed ISO 9001, ISO 13485 and GMP systems. We were awarded CE, FDA, KFDA and PMDA certificates, and earned the honorary titles of a "Unicorn" and "Gazelle" company. We have also undertaken the commercialization projects of scientific and technological achievements from our government. Our products are used in dozens of countries and we have customers on every continent.
We devote ourselves to developing excellent products, strive to be an international leading enterprise in the intervention field and are fully committed to contributing to human well-being!
Our Mission: Develop superb medical devices for people's health and benefits.
Our Vision: Innovation for systematic clinical solutions, and a world-wide intervention leader.
Our Values: Be Pragmatic, Efficient, Collaborative, Willing to share, Hardworking and Dedication.







Certificate

Our Service

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





