Ptca Nickeltitanium Wire Core Medical Guidewire with FDA/CE
Shenzhen Wwks Co Ltd / 2022-06-23

- Type:Surgical Supplies Materials
- Material:Nickeltitanium Wire Core
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:SW
- Transport Package:Cardboard Box
- Trademark:OEM
- Origin:China
- Production Capacity:50000PCS,Month
=== Description ===
Basic Info.
Model NO. SW Transport Package Cardboard Box Trademark OEM Origin China Production Capacity 50000PCS/MonthProduct Description
Product Indication:It is used to guide catheters into blood vessels and position flexible instruments, not suitable for neurovascular.
Product Features:
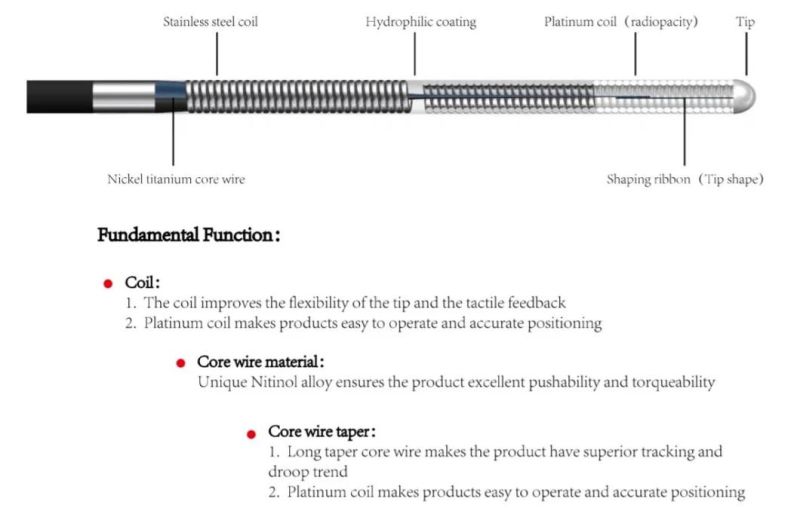
1. Winding wire:
The winding wire improves the flexibility and tactile feedback of the product head end.
The platinum wire is wound to increase the visibility and accurate positioning.
2. Core wire material: nickel-titanium alloy guarantees good pushability and torsion control of the product
3. Core taper:
The long taper core wire makes the product have good traceability.
The short taper core wire makes the product have sufficient support and pushability.
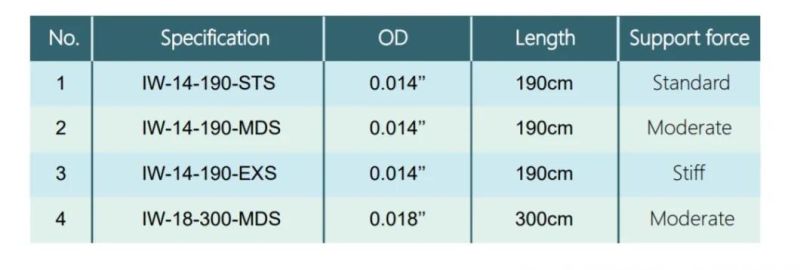
Specification:
Directions For Use
1. Carefully insert the guidewire through the guidewire lumen hub of the interventional device.
2. Advance the guidewire until its tip is just proximal to the interventional device tip.
3. If using a guiding catheter, engage the guiding catheter and insert the interventional device/ guidewire assembly through the hemostatic valve. Advance the system through the guiding catheter until it is just proximal to the tip of the guiding catheter.
4. Tighten the hemostatic valve to create a seal around the interventional device. Ensure intentional guidewire movement is still permitted.
5. Attach the torque device to the guidewire, if desired.
6. Under fluoroscopy, advance the guidewire out of the interventional device while securing the interventional device in place. Use the torque device to steer the guidewire across the lesion.
7. Secure the guidewire in place while tracking the interventional device over it and to the target lesion.
8. If a different tip configuration or guidewire is indicated, carefully remove the guidewire while observing guidewire movement under fluoroscopy.
9. Reshape the guidewire tip according to standard practice or prepare another guidewire to be used.
10. Reinsert the guidewire following Steps 1 through 7 of this section.
Product Performance:
The guide wire has a 1:1 torqueability
The tip of the guidewire is clearly visible under X-ray
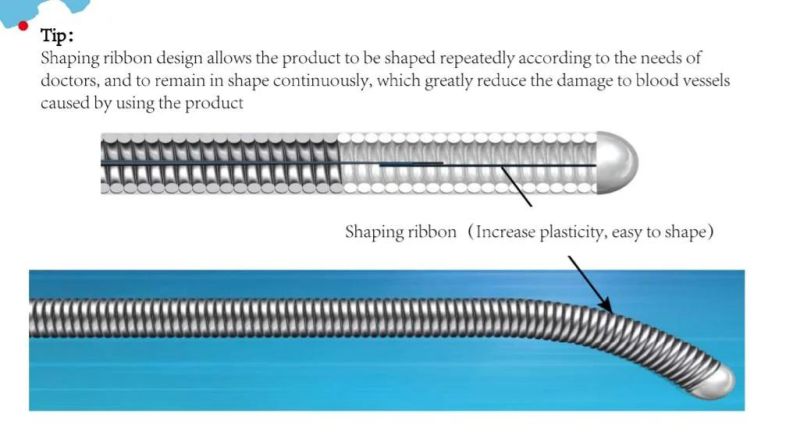
The tip of the guidewire can be shaped repeatedly accordin to the doctor's needs
Stable and superior hydrophilic coating allows products to move freely in blood vessels
Shipping:
Express, Air, sea shipping is available

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





