Disposable CE Certification Biomaterials Anti Adhesive Gel Hyaluronic Acid for Surgical Use
XIAMEN POPTRIMS TEXTILE CO.,LTD. / 2022-06-23
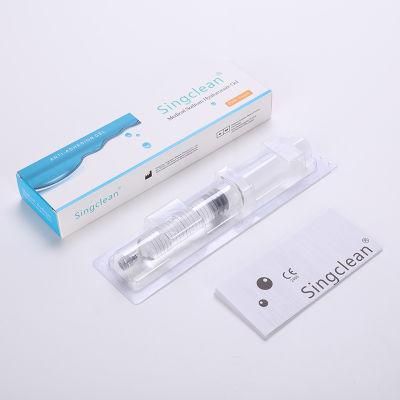
- Material:Hyaluronic Acid Gel
- Feature:Disposable
- Certification:CE, ISO13485, Sfda
- Application:Hospital
- Group:Human
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:Anti Adhesive Gel Hyaluronic Acid Gel
- Hyaluronic Acid:Cross-Linked Derivative
- Department:Pelvic Surgery
- Orginal:Animal Free
- Another Name:Human Wound Repair
- Function:Anti-Adhesion
- Characterist:Better Biocompatibility
- Storage:2-20 C Degree
- Use:Surgical Operation Ends Catheter Into The Uterine
- Company:Manufacture
- Doctor:Obstetrics and Gynecology
- Transport Package:Blister in Color Box
- Specification:5.0ml 10mg,ml
- Trademark:Singclean
- Origin:China
- HS Code:3006700000
- Production Capacity:50000PCS,Year
=== Description ===
Overview
Product Description
Detailed Photos
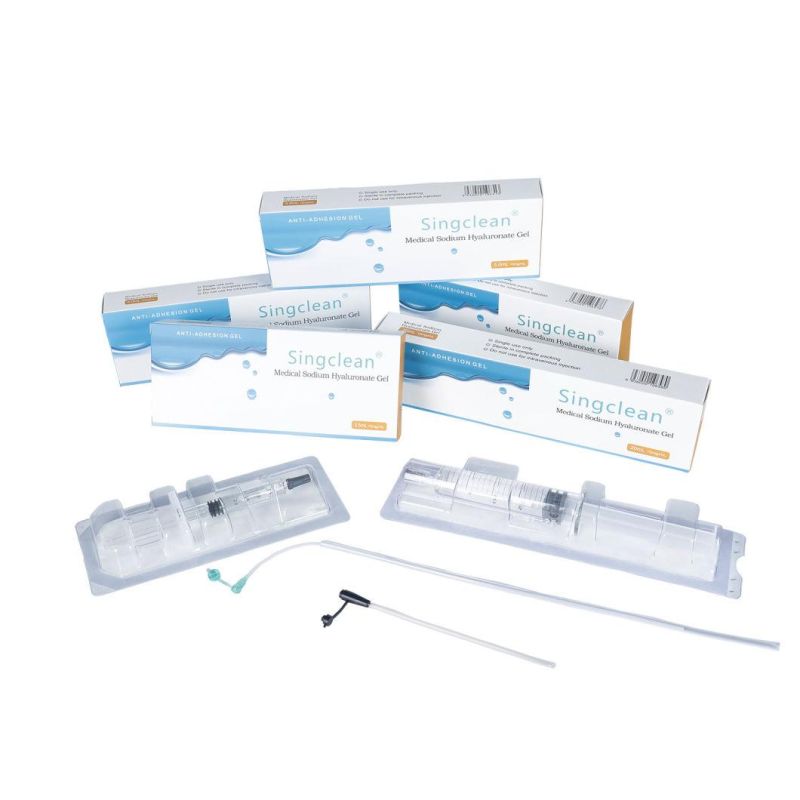

Main Products



New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





