CE/FDA Approved Disposable Closed Suction Catheter for Surgical or Hospital Use
ZHENGZHOU KING PACK LONG MACHINERY CO., LTD. / 2022-06-23

- Material:PVC
- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:All Persons
=== Base Info ===
- Logo Printing:With Logo Printing
- Single Use:Yes
- Character:Closed
- Price:Competitive
- Quality:Excellent
- Lead Time:Fsat
- Transport Package:Packed in Carton, Shipped by Sea, Air, Express
- Trademark:OEM, ODM
- Origin:China
- HS Code:9018390000
- Production Capacity:1000000000000PCS,Year
=== Description ===
Basic Info.
Logo Printing With Logo Printing Single Use Yes Character Closed Price Competitive Quality Excellent Lead Time Fsat Transport Package Packed in Carton, Shipped by Sea, Air, Express Trademark OEM, ODM Origin China HS Code 9018390000 Production Capacity 1000000000000PCS/YearProduct Description
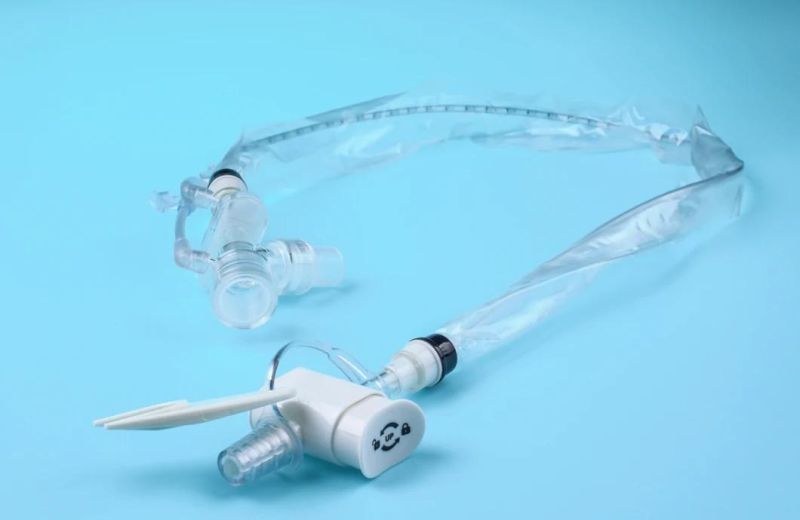
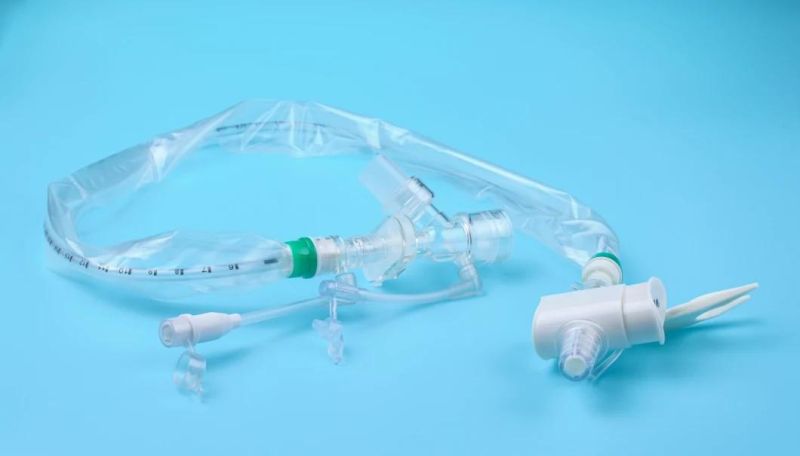
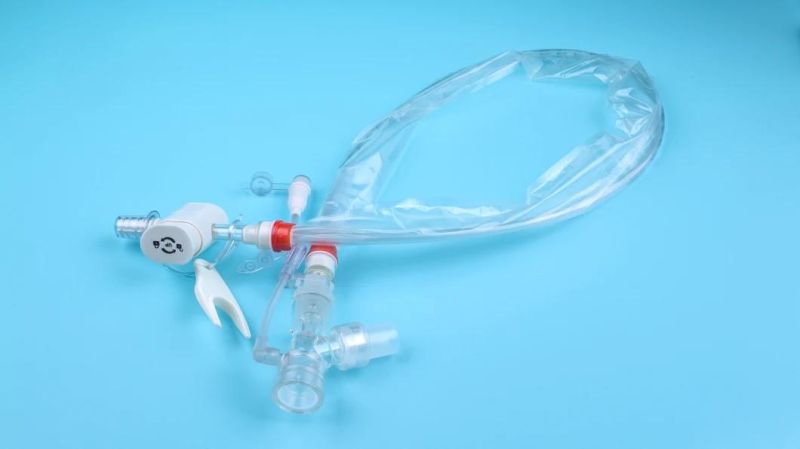
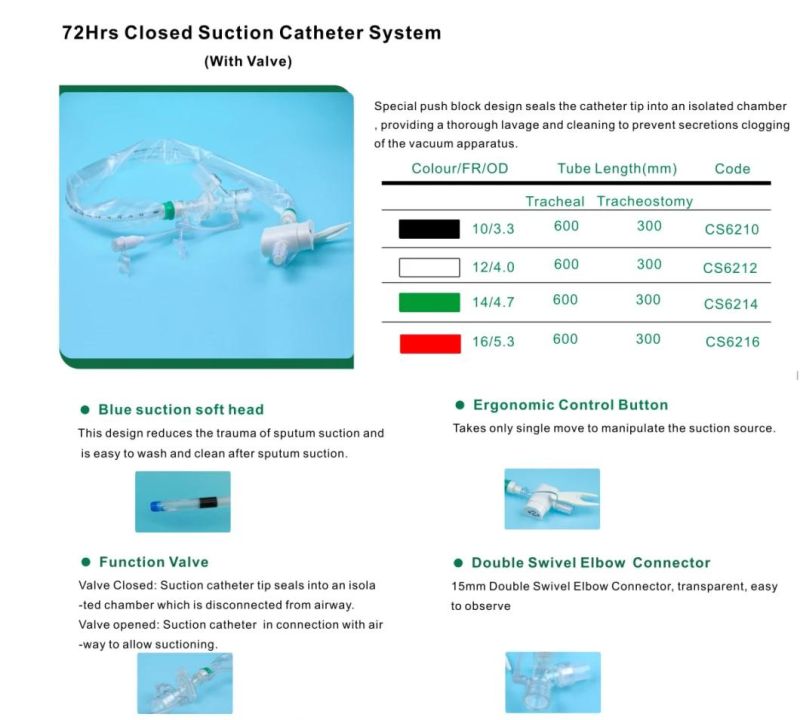
The utility model is suitable for patients with respiratory diseases, general anesthesia operation, emergency treatment, etc. the utility model needs to be used for tracheal intubation (or tracheotomy intubation).
The catheter is enclosed in a plastic sleeve. It attaches between the ventilator circuit and the tracheostomy tube.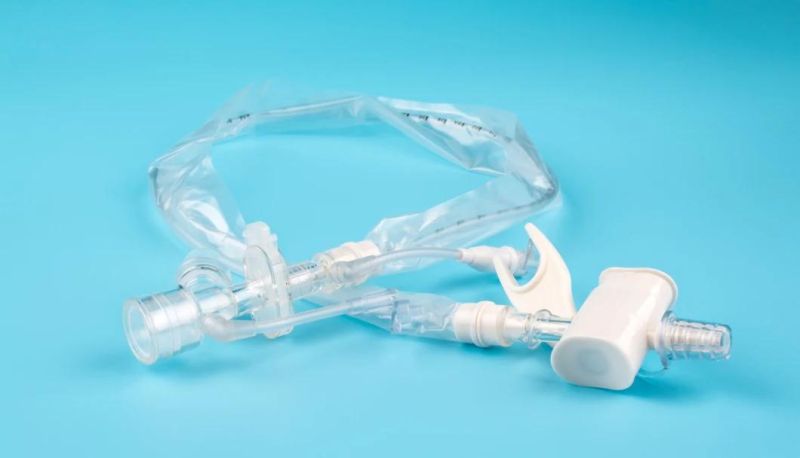

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





