Disposable Laparoscopic Trocars Factory Best Selling 12mm Trocars for Endoscopic Procedures
Hebei Zhouwo Trading Co., Ltd. / 2022-06-23

- Type:Surgical Supplies Materials
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:3 Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:T12-100-CT-F
- Transport Package:Blister Package in Carton Case
- Specification:12mm*100mm
- Trademark:TK
- Origin:China
- HS Code:90189099
- Production Capacity:200000 Pieces,Year
=== Description ===
Basic Info.
Model NO. T12-100-CT-F Transport Package Blister Package in Carton Case Specification 12mm*100mm Trademark TK Origin China HS Code 90189099 Production Capacity 200000 Pieces/YearProduct Description
Disposable laparoscopic trocars factory best selling 12mm trocars for endoscopic proceduresGTK Medical, established on March 3, 2004, is a hi-tech medical device manufacturer which focuses on the R&D and manufacturing surgical devices like trocars, retrieval bags for more than 17 years.
GTK Medical devices are exported to more than 40 countries including but not limited to the UK, US, Germany, France, Italy, Spain, Belgium, Japan, South Korea etc.


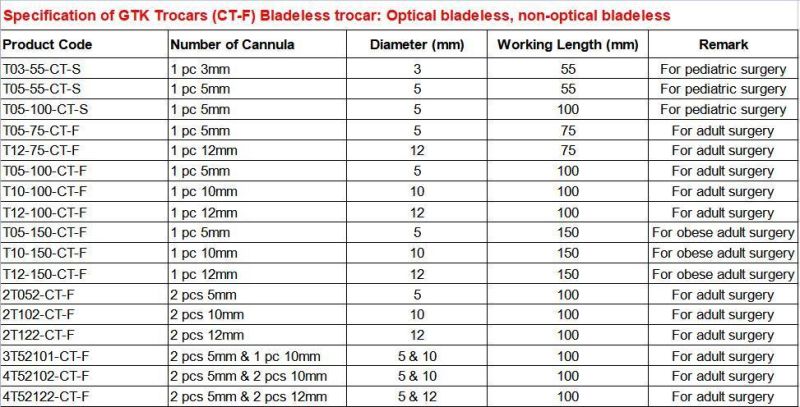
GTK Trocars specifcations meet the needs of all clinical demands;
GTK Medical passed GMP of NMPA(CHINA FDA) AND US FDA factory registration (No. 3008191256) and ISO13485:2016 medical devices quality system.
GTK Medical products including trocars are used in more than 1000 hospitals throughout the world.




Q&A on GTK Trocars;
1. Q: What is the core strength of GTK Medical?
A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products.
2. Q: What makes GTK Trocars stand out?
A: Excellent sealing performance plus excellent smooth insertion and removal of surgical instruments.
3. Q: Do you provide free samples of GTK Trocars for clinical trial use?
A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation.
4. Q: Do you have exported experiences to large medical devices company/companies?
A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe.
5. Q: Do you accept factory audit prior to formal partnership?
A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021.
Send me an inquiry, I will provide you with best product and warm services.
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





