Sterile Surgical Sutures (absorbable and nonabsorbable)
Safecare Medical Products Co., Ltd.(Hefei) / 2022-06-23
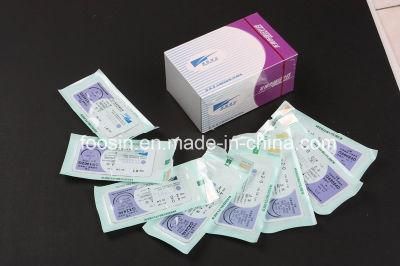
- Type:Surgical Supplies Materials
- Material:Polyglycolic Acid
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:All
- Logo Printing:Without Logo Printing
=== Base Info ===
- Model NO.:PGA, PDO, PGLA, PGCL, Nylon, Silk, Polyester, PP
- Package:12 Pieces,Box
- Carton:100 Boxes , Carton
- Transport Package:1 Piece,Packet
- Specification:CE, ISO
- Trademark:Wegosutures
- Origin:China, Weihai
- HS Code:9018322000
- Production Capacity:15, 000, 000sutures,Year
=== Description ===
Basic Info.
Model NO. PGA, PDO, PGLA, PGCL, Nylon, Silk, Polyester, PP Package 12 Pieces/Box Carton 100 Boxes / Carton Transport Package 1 Piece/Packet Specification CE, ISO Trademark Wegosutures Origin China, Weihai HS Code 9018322000 Production Capacity 15, 000, 000sutures/YearProduct Description

WEGO HOLDING COMPANY LIMITED ("WEGO") established in March 1988, and the major business of WEGO focus on medical devices and pharmaceuticals. In addition, WEGO develops some other business such as real estate, investment and so on. WEGO has 9 industry groups and more than 30 subsidiaries. The main products of WEGO are infusion set& syringe, blood transfusion equipment, heart stent & intracardiac consumable,IV catheter & special needle, blood purification equipment and consumables, orthopedic material, operation room equipment and accessories, wound care product, minimally invasive instrument and equipment, ICU product and accessories, high-capacity injection and pharmaceutical, renal product, biological diagnostic reagent, PVC & non-PVC material and so on. WEGO produces more than 30 series, 400 categories and 40,000 specifications products, and has become one of the most reliable suppliers of medical system in the word. At the same time, WEGO energetically takes part in healthcare service and provides renal dialysis service. WEGO's holding subsidiary ---SHANDONG WEIGAO GROUP MEDICAL POLYMER CO., LIMITED is listed in Hong Kong.
Foosin Medical Supplies Inc., Ltd, established in 2005, is a joint venture company between Wego Group and Hong Kong, with total capital over RMB 50 million. We are trying to contribute to make Foosin become to the most powerful manufacture base of surgical needle and surgical sutures in the develop countries. Main product covers Surgical Sutures, Surgical Needles and Dressings.
R&D
Fully introduced the advance world level inspection devices from U.S, keep a foothold in the domestic to developing the world-leading technique and technology which have many patents at present and to be the provincial high-tech enterprises.
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center.
WEGO-PGA Features
Structure Multifilament, braided
Chemical composition 100%polyglycolic acid
Coating Polycaprolactone + Calcium Stearate
Color Violet or Undyed
Sizes USP 2-USP 8/0 metric 5 - metric 0.4
Knot tensile strength Retention 14 days post implantation 60-70%
18 days post implantation 50%
21 days post implantation 40%
Mass absorption Degradation by hydrolysis within 60-90 days
Indications General surgery, Gastroenterology, Urology, Gynaecology, Ophthalmic, Plastic and Paedriatic surgery
Sterilization Ethylene oxide
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





