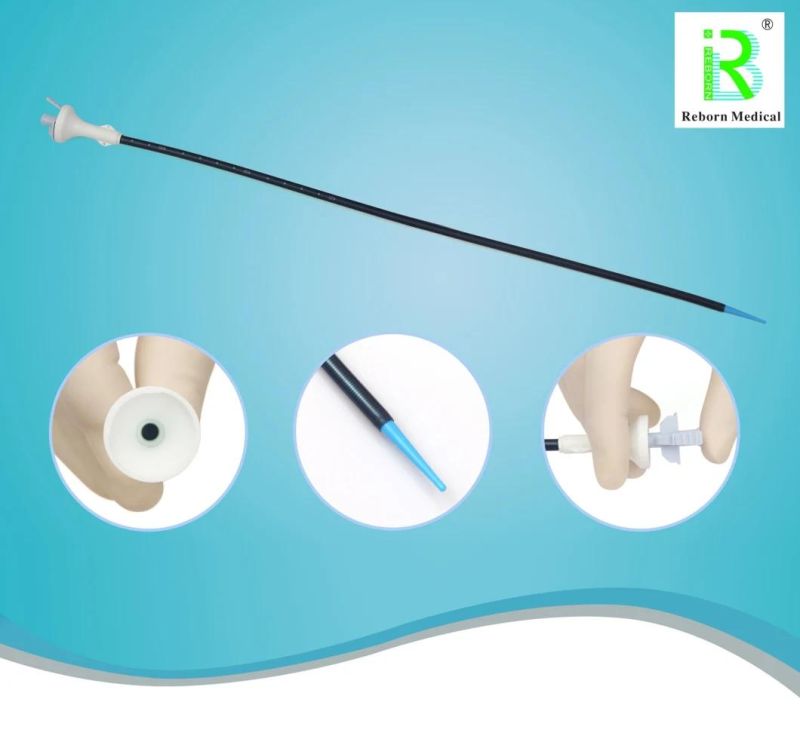
Reborn Medical Ureteral Access Sheath Hydrophilic F10-F14 with CE Certificate
Rifeng Hardware and Mould Making Co., Ltd. / 2022-06-23

- Type:Sheath
- Material:PTFE PT Hydrophilic Stainless Steel Wire
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adults and Children
- Logo Printing:with Logo or Without Logo
=== Base Info ===
- Model NO.:reborn-UAS
- Color:Black and Blue
- Length:20-55cm
- Certificate:CE,ISO
- After-Sales:12 Hours Online
- Application:Hospital
- Sample:Available
- Export Market:Global
- Feature:Disposable
- Transport Package:Plastic Package and Carton Package
- Specification:F10 F11 F12 F13 F14
- Trademark:Reborn
- Origin:China
- HS Code:9018390000
- Production Capacity:50000 Pieces,Year
=== Description ===
strengthening wire is equipped inside the sheath with terrific foldability and pressure resisitance ,ensuring surgical instruments smooth operation in the sheath.
tapered tip is designed for atraumatic advancement.
It is used for urological flexible ureteroscope to establish surgical
access, to assist endoscope and other instruments to enter the
urinary tract, to provide a continuous operation channel, to protect
the ureter when instruments are repeatedly exchanged, to reduce
the possibility of trauma, and to protect Precision instruments and
soft mirrors are protected from damage.
about reborn
The factory is located in Zhuzhou (National) High-tech Industrial
Development Zone with beautiful scenery and convenient transportation.
The company regards product quality as its life and strictly implements
total quality management. Aseptic products are produced in 100,000-level
purification workshops, with a purification area of nearly 1,500 square meters.
In order to ensure the hygiene requirements of the products, and equipped
with professional laboratories and testing instruments, the trained quality
inspectors strictly control the quality, are meticulous in product quality,
and strive for excellence. All products are produced in strict accordance
with the standardization of the quality management system.
The main products produced by the company are consumables for urological
surgery, including J-type catheter, nephrostomy catheter, bladder fistula catheter,
ureteral catheter and ureteral balloon catheter, minimally invasive dilatation and
drainage kit, flexible endoscope introducing sheath, guide wire, stone retrieval
basket, etc. , The product material is all imported materials.

Storage Condition:
- Put them in airy and dry places and avoid the exposure of corrosive gas
- Less than 40 centigrade and keep the humidity between 30%-80%
- Pay attention to mice, insects and package damages.

Reborn Medical Equipment Co.,Ltd
1. Reliable customer service
2. Competitive and moderate price
3. Trust worthy
4. Large market share both at home and abroad
5. OEM and ODM cooperation all over the world
6. Strict quality control
7. Ethylene oxide sterilization
8.We treat clients as friend
9.We special focus on after-sale service
10.Welcome to visit our factory in China
Contact Information:
Name:grace
Website: reborn-medical.en.made-in-china.com
Address: Zhuzhou City, Hunan Province, China
All of specification and length can be customized according to clinical needs
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





