FDA Certified Single Use Disposable Exsanguination Tourniquet Non Pneumatic Non Power Factory
Yiwu Bokun Adhesive Tape Co., Ltd. / 2022-06-23

- Certification:FDA
- Feature:Disposable
- Color:Cyan, Green, Blue, Pink
- Type:Cloth Tourniquet
- Max Wearing Time:90 Minutes
- Expiry Date:2 Years
=== Base Info ===
- Transport Package:1PC,Box, 100box,Carton
- Specification:19.0*7.0*15.0 (cm)
- Trademark:E. R. A
- Origin:China
- Production Capacity:5000000 Pieces,Year
=== Description ===
Lower Limb
 1.3 Product composition: An elastic silicone ring, an elastic stockinet sleeve, two colored pulling straps, two handles, a binder tie, and a plastic card.
1.3 Product composition: An elastic silicone ring, an elastic stockinet sleeve, two colored pulling straps, two handles, a binder tie, and a plastic card.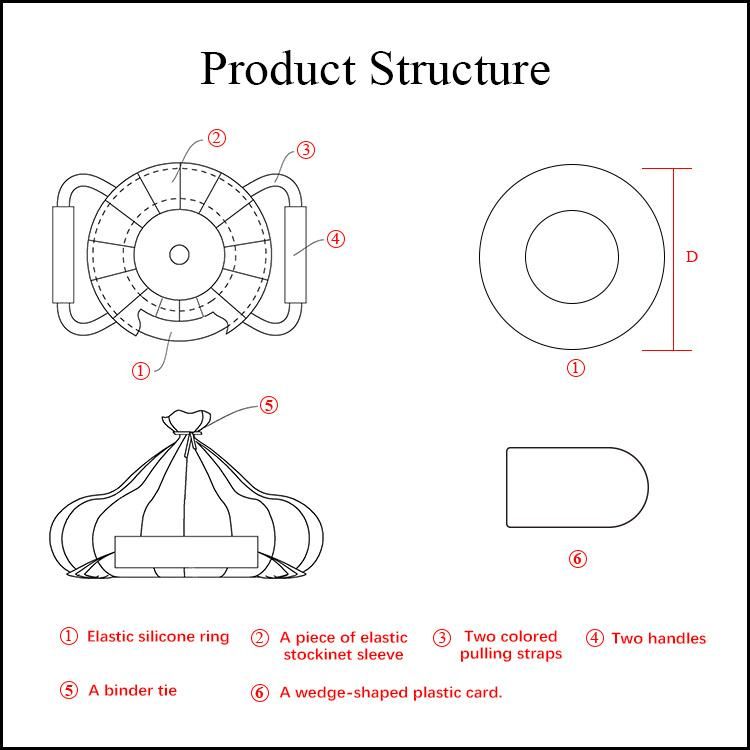
2. Residual Ethylene Oxide
The product is sterilized by ethylene oxide, and the residual amount of ethylene oxide should not exceed 10 µg/g.
3. Application Field
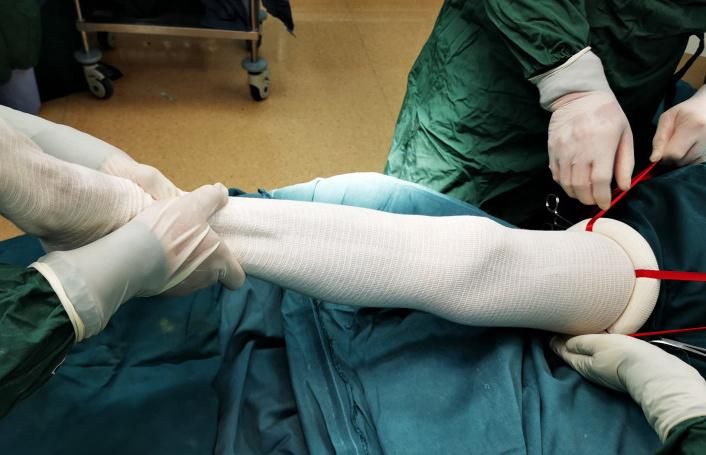
Limb surgeries that require exsanguination and occlusion.
4. Contraindications
4.1 Severe skin damage on limbs.
4.2 Insufficient peripheral blood flow, edema, or deep vein thrombosis (DVT).
4.3 Limb infections or malignant tumors.
4.4 Heart disease and respiratory disorders that were caused by exsanguination devices.
5. Precautions and suggestive instructions
5.1 Potential hazards and restrictions on use
This product is a single-use device. After the expiration date, it cannot be used normally, and after use, it should be disposed of in accordance with the country's existing medical waste safety management regulations.
This product is sterilized by ethylene oxide. If the inner packaging is damaged, please do not use it.
If the product cannot block the blood flow of the patient's limbs, it should be removed immediately.
The hemostasis time of this product should not exceed 90 minutes.
Do not place the elastic ring of this product on the elbow (where it is likely to cause damage to the ulnar nerve) or the knee (where it is likely to cause damage to the common peroneal nerve).
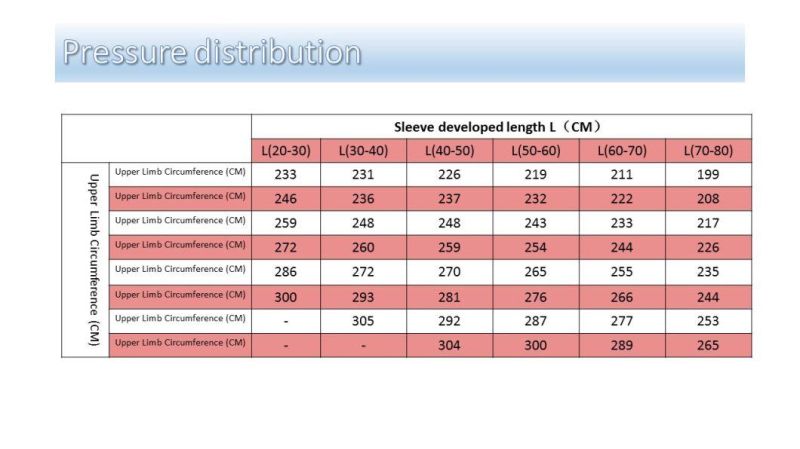
Confirm that the circumference of the mid-upper part of the patient's upper arm is between 14-40cm and the middle thigh between 28-85cm. If the measured circumference exceeds the above range, please use other methods to conduct hemostasis. If the systolic blood pressure is higher than 180mmHg, please take other methods to conduct hemostasis.
5.2 It is recommended to use the device when the patient is under anesthesia. If not, the patient will feel uncomfortable. Explain to the patient before use.
6. Warnings
6.1 Possible adverse reactions
Numbness, edema, and hematoma at the site of hemostasis.
Skin ecchymosis, blisters and necrosis.
Ineffective hemostasis and worsening bleeding.
Tourniquet pain.
Tourniquet shock.
Muscle nerve injury.
Vascular damage, thrombosis.
Other complications of combined surgery.
6.2 The elastic contraction ring of this product must not roll to the distal end of the limb after reaching the working part.
7. Symbels
8. Instructions
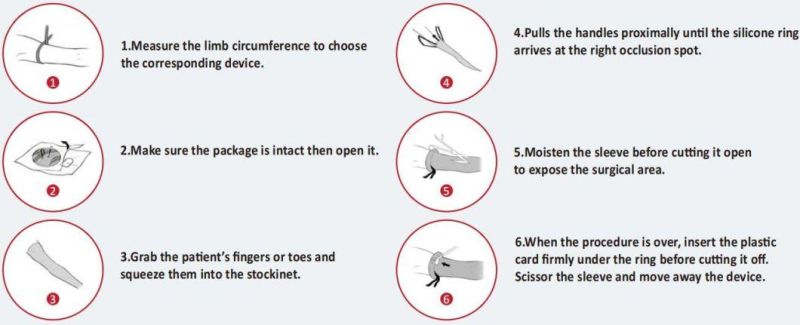
9. Storage and transportation conditions
9.1 Storage: The product should be placed in a clean room with a relative humidity of no more than 80% RH, no corrosive gas, and good ventilation.
9.2 Transportation: During transportation, the packaging must neither be bumped nor be exposed to rain.
10. Technical Principles
The diameter of the elastic ring is smaller than the minimum size of the limb (even the extremity). When the limb is inserted into the ring, the ring will be expanded, therefore exerts a counter-effect on the limb, squeezing the blood out of vessels. In order to generate enough force to resist the blood pressure, in the meantime not to generate excessive force to damage the tissues, the material of the ring must be selected reasonably.

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





