One Step Rapid Nucleic Acid Extraction and Release Agent for PCR Machine
Dongguan Jiacheng Packaging Material Co., Ltd. / 2022-06-23

- Type:Rna,DNA Extraction Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Without Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:All People
- Logo Printing:With Logo Printing
=== Base Info ===
- Model NO.:AG-RLS003-500ul
- Usage:Specimen Release Agent-Nucleic Acid Specimen Auto
- Period Usage:Specimen Pre-Treatment for DNA, Rna
- Proceed:Samples Processed by This Product Can Be PCR Templ
- Under Temperature:Room Temperature
- Type of Specimen:Lyse Pathogens
- Equipments Related:No Need Heating, Centrifuging or Replacing Tubes
- Type of Gene:The Sample of DNA,Rna Can Be Extracted
- Processing:Simple Operationsn
- Processing Time:5-10 Minute Stand
- PCR Steps:for The Pretreatment of Nucleic Acids
- Specimen After Doing Release Process:Clinical in Vitro Diagnosis or Used for The Detect
- Bottle Type:Drip Type
- Transport Package:Packed in Tube, Boxes Collected in Carton.
- Specification:one-tube fast release technology platform
- Origin:Guangzhou
- Production Capacity:5000000 a Week
=== Description ===
Basic Info.
Model NO. AG-RLS003-500ul Usage Specimen Release Agent-Nucleic Acid Specimen Auto Period Usage Specimen Pre-Treatment for DNA, Rna Proceed Samples Processed by This Product Can Be PCR Templ Under Temperature Room Temperature Type of Specimen Lyse Pathogens Equipments Related No Need Heating, Centrifuging or Replacing Tubes Type of Gene The Sample of DNA/Rna Can Be Extracted Processing Simple Operationsn Processing Time 5-10 Minute Stand PCR Steps for The Pretreatment of Nucleic Acids Specimen After Doing Release Process Clinical in Vitro Diagnosis or Used for The Detect Bottle Type Drip Type Transport Package Packed in Tube, Boxes Collected in Carton. Specification one-tube fast release technology platform Origin Guangzhou Production Capacity 5000000 a WeekProduct Description
PRODUCT NAMESpecimen Release Agent
PACKAGE SPECIFICATION
500µL/Tube, 50 Tubes/Kit;
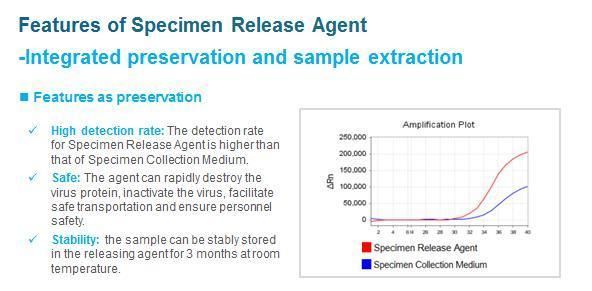
Sample release reagent developed based on the Sansure one-tube fast release technology platform. Adopting Sansure nucleic acid release technology, can quickly lyse pathogens at room temperature, no need heating, centrifuging or replacing tubes, the sample DNA/RNA can be extracted quickly through simple operations.
Sample Release Reagent is used for the pretreatment of nucleic acids, to release the nucleic acids from specimens, then the released nucleic acids can be used for clinical in vitro diagnosis or used for the detection through equipment.
INTENDED USE
This product is intended for specimen pre-treatment, so that the test substance in the specimen is released from the state of being combined with other substances, to facilitate the use of in vitro diagnostic reagents or instruments to test the substance.
PRINCIPLE
This product can denature the protein structure of cells or viruses, and lyse specimens to release nucleic acids. This product does not affect the subsequent nucleic acid analysis and does not inhibit the PCR reaction.
The components of this product have the effect of inactivating viruses, but biological samples of major infectious diseases should be inactivated strictly in accordance with relevant guidelines and strictly operated in a biological safety cabinet.
MAIN COMPONENTS
This product is mainly composed of Tris buffer, Sodium dodecyl sulfate and Sodium chloride.
STORAGE CONDITIONS AND SHELF LIFE
Store at room temperature, valid for 24 months. Production date, expiration date: see packaging label.

APPLICATION
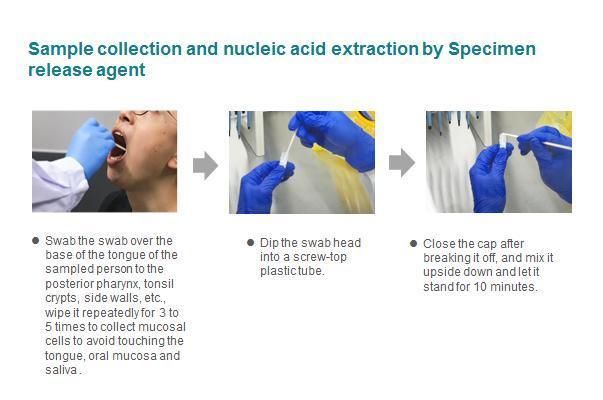
This product is for nasopharyngeal swab, oropharyngeal swab specimens or specimens stored in RNase-free water / saline / PBS buffer, not for specimens stored in preservation solutions containing guanidine salts or PCR inhibitors.

*Note: When it use as sample extraction, this product is for specimens stored in RNase-free water /saline / PBS buffer, not for specimens stored in preservation solutions containing guanidine salts or PCR inhibitors.
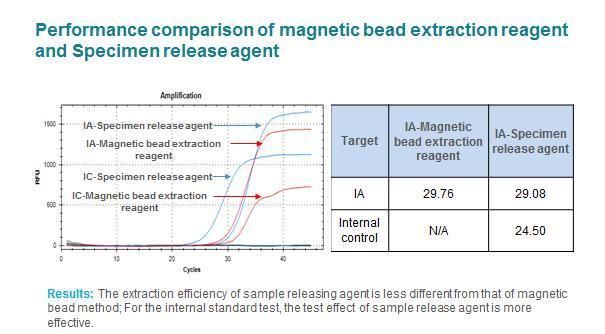
Samples processed by this product can be PCR templates for nucleic acid test kit (e.g. Detection Kit for RNA (PCRFluorescence Probing) produced by Da An Gene Co., Ltd. Of Sun Yat-sen University; RNA+DNA Nucleic Acid Diagnostic Kit produced by Sansure BioTech Inc.). It should be noted that the processed sample is added as a template to the nucleic acid detection reagents of various manufacturers, and should be shaken and mixed and centrifuged for PCR amplification. Samples processed by this product can also be used for nucleic acid extraction or purification kit of various methodologies widely used in the market, of which magnetic bead and spin column method have been verified.
Easy opearation
More accuracy, compare to magnetic bead extraction reagent.
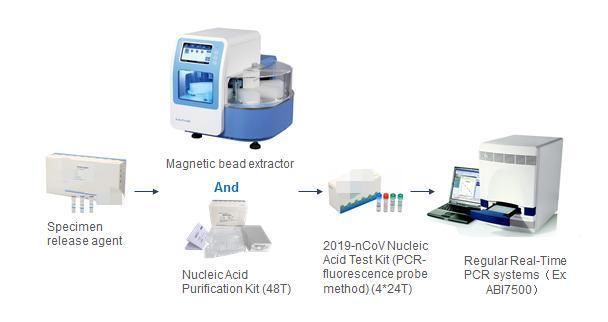
Whole work flow for PCR when using Specimen release agent.
New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





