Sterilized Disposable Elastic Tourniquet Exsanguination Silicone Ring for Limb Procedures Blood Occlusion/Removal Safe Efficient Orthopedic Surgical Accessory
Yiwu Bokun Adhesive Tape Co., Ltd. / 2022-06-23
- Customized:Non-Customized
- Feature:Disposable
- Color:White Ring, Colorful Straps
- Type:Cloth Tourniquet
- Expiry Date:3 Years
- Max Wearing Time:90 Minutes
=== Base Info ===
- Material:Silicone and Nylon
- Transport Package:1PC,Box, 100box,Carton
- Specification:200*200(mm)
- Trademark:E. R. A
- Origin:China
- Production Capacity:5000000 Pieces,Year
=== Description ===
Lower Limb BLD-QZX-XZ
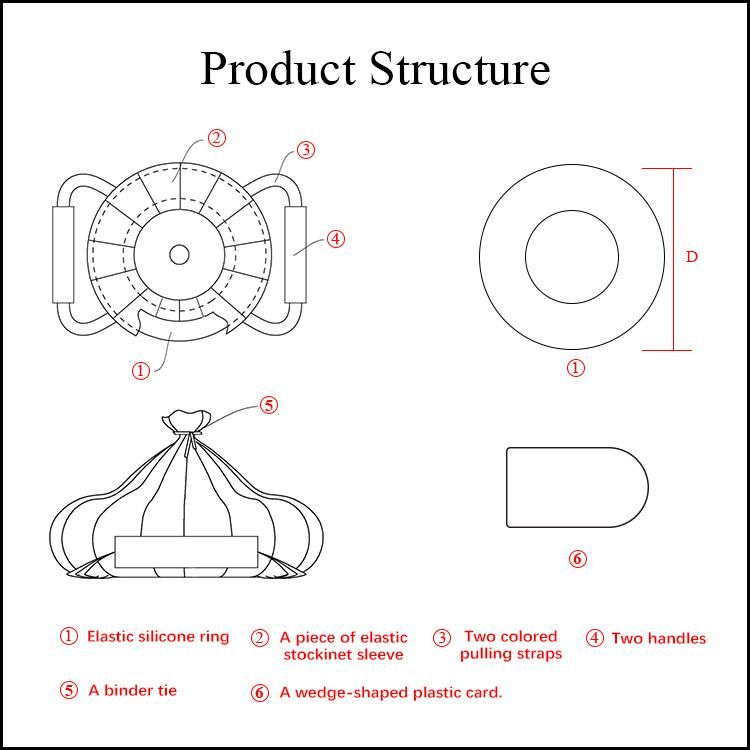
1.3 Product composition: An elastic silicone ring, an elastic stockinet sleeve, two colored pulling straps, two handles, a binder tie, and a plastic card.
2. Main performance index
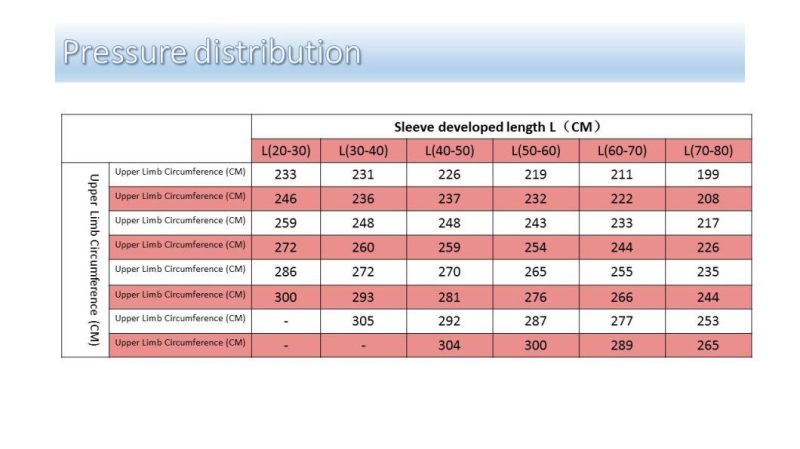
2.1 Size
2.1.1 The outer diameter D of the elastic ring of the upper limb type (except model L1500) is between 77±4mm; that of L1500 is between 57±4mm.
2.1.2 The outer diameter D of the elastic ring of the lower limb type (except model L2400, L2500, L2600) is between 99±5mm; that of L2700 and L2800 is between 104±5mm.
2.1.3 The thickness of the plastic card is between 0.8±0.1mm.
2.2 Appearance
2.2.1 The device should be clean, and free of dirt or damage.
2.2.2 Before use, the elastic sleeve is wrapped around the elastic ring, and the shape of the elastic sleeve after unfolding is like a sock tube.
2.3 Tensile Force
2.3.1 The elastic sleeve of the upper limb type should be able to withstand a force of 150N without damage.
2.3.2 The elastic sleeve of the lower limb type should be able to withstand a force of 150N without damage.
2.3.3 A single pulling strap of the upper limb type should be able to withstand a force of 150N without breaking.
2.3.4 The binder ties should be able to withstand a force of 150N after being locked, without breaking or tripping.
2.4 Strength
2.4.1 The elastic ring of the upper limb type should be stretched to 200mm without breaking.
2.4.2 The elastic ring of the lower limb type should be stretched to 200mm without breaking.
2.5 Sterilization
The product is delivered in a sterile state and should be sterile.
2.6 Residual Ethylene Oxide
The product is sterilized by ethylene oxide, and the residual amount of ethylene oxide should not exceed 10 µg/g.
3. Application Field
Limb surgeries that require exsanguination and occlusion.
4. Contraindications
4.1 Patients with severe skin damage on limbs.
4.2 Patients with insufficient peripheral blood flow, edema, or deep vein thrombosis (DVT).
4.3 Patients with limb infections or malignant tumors.
4.4 Patients who have had heart disease and respiratory disorders that were caused by exsanguination devices.
5. Precautions and suggestive instructions
5.1 Potential hazards and restrictions on use
5.1.1 This product is a single-use device. After the expiration date, it cannot be used normally, and after use, it should be disposed of in accordance with the country's existing medical waste safety management regulations.
5.1.2 This product is sterilized by ethylene oxide. If the inner packaging is damaged, please do not use it.
5.1.3 If the product cannot block the blood flow of the patient's limbs, it should be removed immediately.
5.1.4 The hemostasis time of this product should not exceed 90 minutes.
5.1.5 Do not place the elastic ring of this product on the elbow (where it is likely to cause damage to the ulnar nerve) or the knee (where it is likely to cause damage to the common peroneal nerve). Confirm that the circumference of the mid-upper part of the patient's upper arm is between 14-40cm and the middle thigh between 28-85cm. If the measured circumference exceeds the above range, please use other methods to conduct hemostasis. If the systolic blood pressure is higher than 180mmHg, please take other methods to conduct hemostasis.
5.2 Before using this product, the operator should read this manual carefully, be familiar with the performance and the use of the device.
5.3 It is recommended to use the device when the patient is under anesthesia. If not, the patient will feel uncomfortable. Explain to the patient before use.
6. Warnings
Possible adverse reactions
6.1.1 Numbness, edema, and hematoma at the site of hemostasis.
6.1.2 Skin ecchymosis, blisters and necrosis.
6.1.3 Ineffective hemostasis and worsening bleeding.
6.1.4 Tourniquet pain.
6.1.5 Tourniquet shock.
6.1.6 Muscle nerve injury.
6.1.7 Vascular damage, thrombosis.
6.1.8 Other complications of combined surgery.
6.2 The elastic contraction ring of this product must not roll to the distal end of the limb after reaching the working part.
7. Symbels
8. Instructions
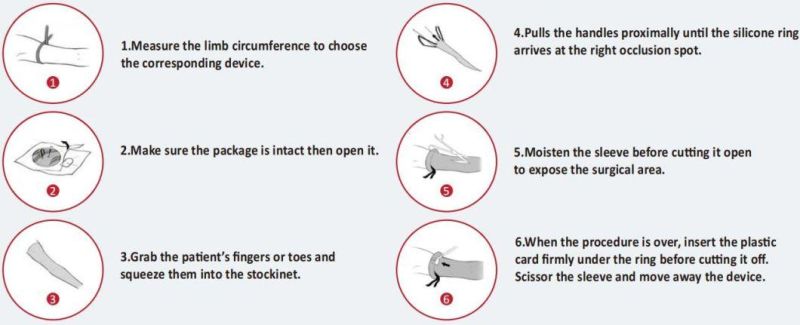
8.1 Measure the circumference of the patient's limb at the exsanguination site (mid-upper part of the upper arm; middle thigh). Confirm that the circumference is within the applicable range of Table 1. If it exceeds the above range, please use other methods to conduct hemostasis.
8.2 Take out the product according to the aseptic procedure in the operating room and then place it on the table. Ensure that the plastic card is available at the end of the operation.
8.3 Disinfect the limb up to the mid-upper part of the upper arm or above the middle thigh depending on the surgical site and use a sterile transparent drape to cover the edge of the disinfected area.
8.4 Product application
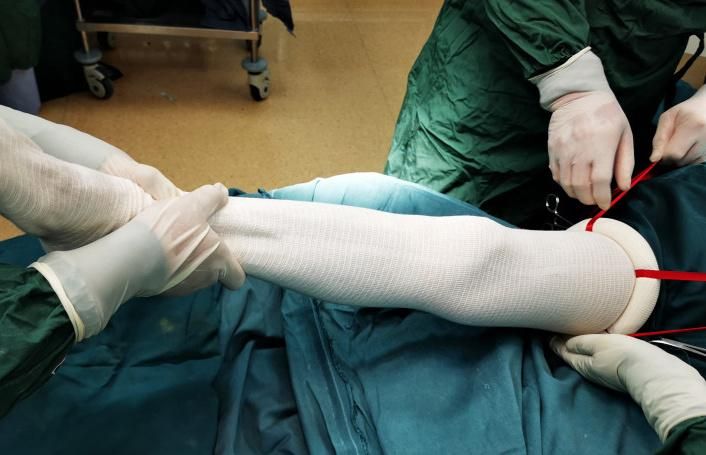
8.4.1 Upper limb type: If the patient is conscious, ask the patient to gather the fingers together. If the patient is unconscious, the assistant (wearing sterile gloves) should hold the patient's fingers together, squeeze them into the ring (the handles facing away from the patient) and pull the handles to the proximal end, while the other assistant (wearing sterile gloves) grasps the end of the limb to fight against the traction, so that the device could scroll up to the proximal end smoothly.
8.4.2 Lower limb type: the assistant (wearing sterile gloves) should squeeze the patient's toes into the ring (the handles facing away from the patient), and pull the handles to the proximal end, while the other assistant (wearing sterile gloves) grasp the end of the limb to fight against the traction so that the device could scroll up to the proximal end smoothly.
8.4.3 Pull the handles until the elastic ring is on the surgical site: 10cm above the wrist joint or the mid-upper part of the upper arm; 5-15cm above the lateral malleolus that is below the calf muscle, or the middle of the thigh. The device should be kept within the disinfection area.
8.5 Use sterile injection water or 0.9% injection sodium chloride to infiltrate the elastic sleeve at the surgical site and then cut it. Wetting the sleeve before cutting can minimize fabric fragments. The surgical site can be covered with a sterile transparent drape.
8.6 When the operation is over, insert the plastic card from the distal end under the elastic ring, and cut the ring with a scalpel. Use scissors to cut up the sleeve and remove it from the patient's limbs. At this time, blood circulation will be resuming. Hemodynamic changes should be paid attention to and treated in time.
9. Storage and transportation conditions
9.1 Storage: The product should be placed in a clean room with a relative humidity of no more than 80% RH, no corrosive gas, and good ventilation.
9.2 Transportation: During transportation, the packaging must neither be bumped nor be exposed to rain.
10. Technical Principles
The diameter of the elastic ring is smaller than the minimum size of the limb (even the extremity). When the limb is inserted into the ring, the ring will be expanded, therefore exerts a counter-effect on the limb, squeezing the blood out of vessels. In order to generate enough force to resist the blood pressure, in the meantime not to generate excessive force to damage the tissues, the material of the ring must be selected reasonably.


New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





