Hyaluronic Acid Filler Injection Injectable Dermal Filler 2ml Ha Filler Lip Breast Filler Hyaluronate
Cangzhou Zhongtian Plastic Products Co., Ltd. / 2022-06-23

- Certification:ISO, CE
- Disinfection:Disinfection
- Color:Transparent
- Application:Chest
- Material:Hyaluronic Acid
- Transport Package:Box
=== Base Info ===
- Model NO.:T1
- Specification:22X11X4 cm
- Trademark:tiantian
- Origin:China
- HS Code:3006700000
- Production Capacity:5000000
=== Description ===
Basic Info.
Model NO. T1 Specification 22X11X4 cm Trademark tiantian Origin China HS Code 3006700000 Production Capacity 5000000Product Description
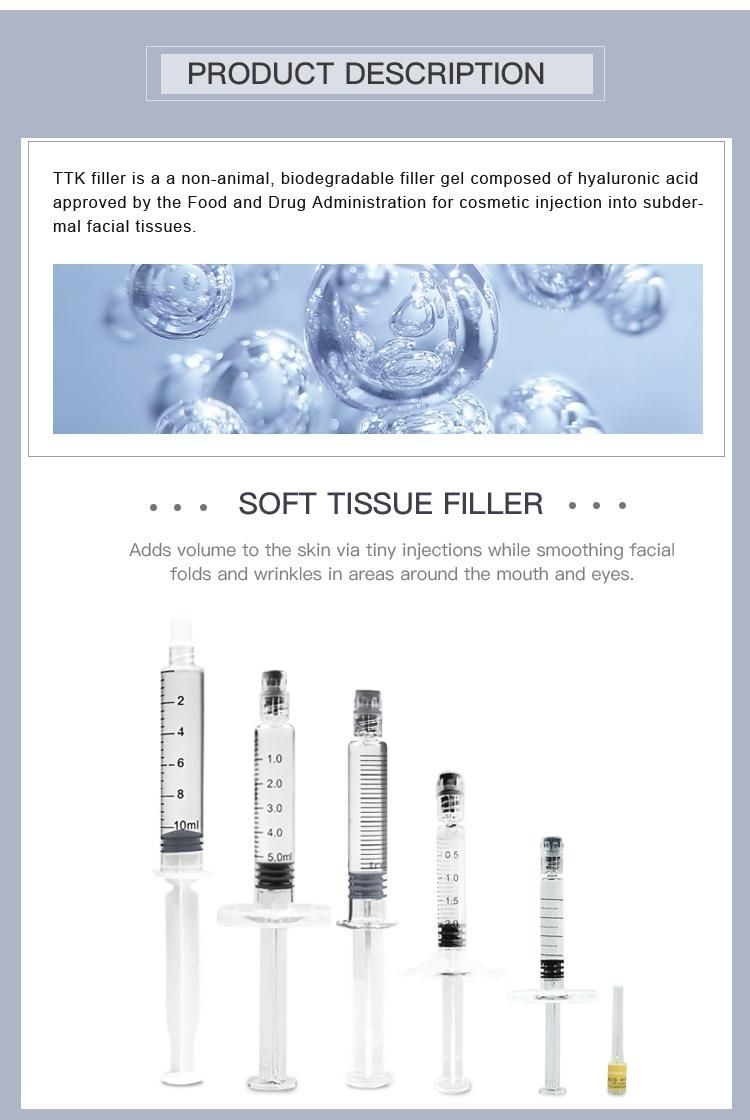
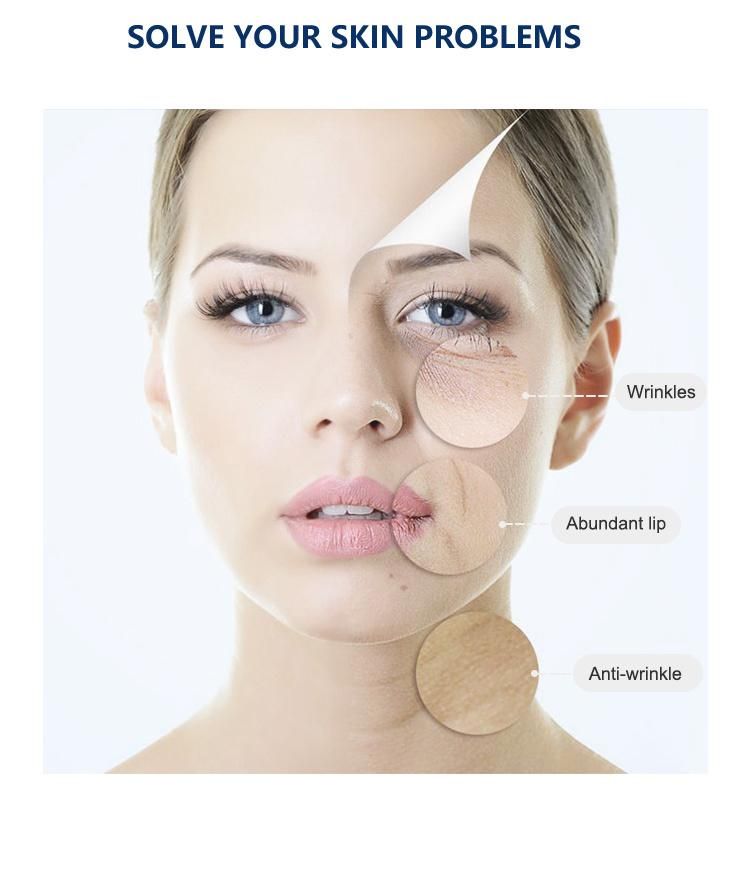
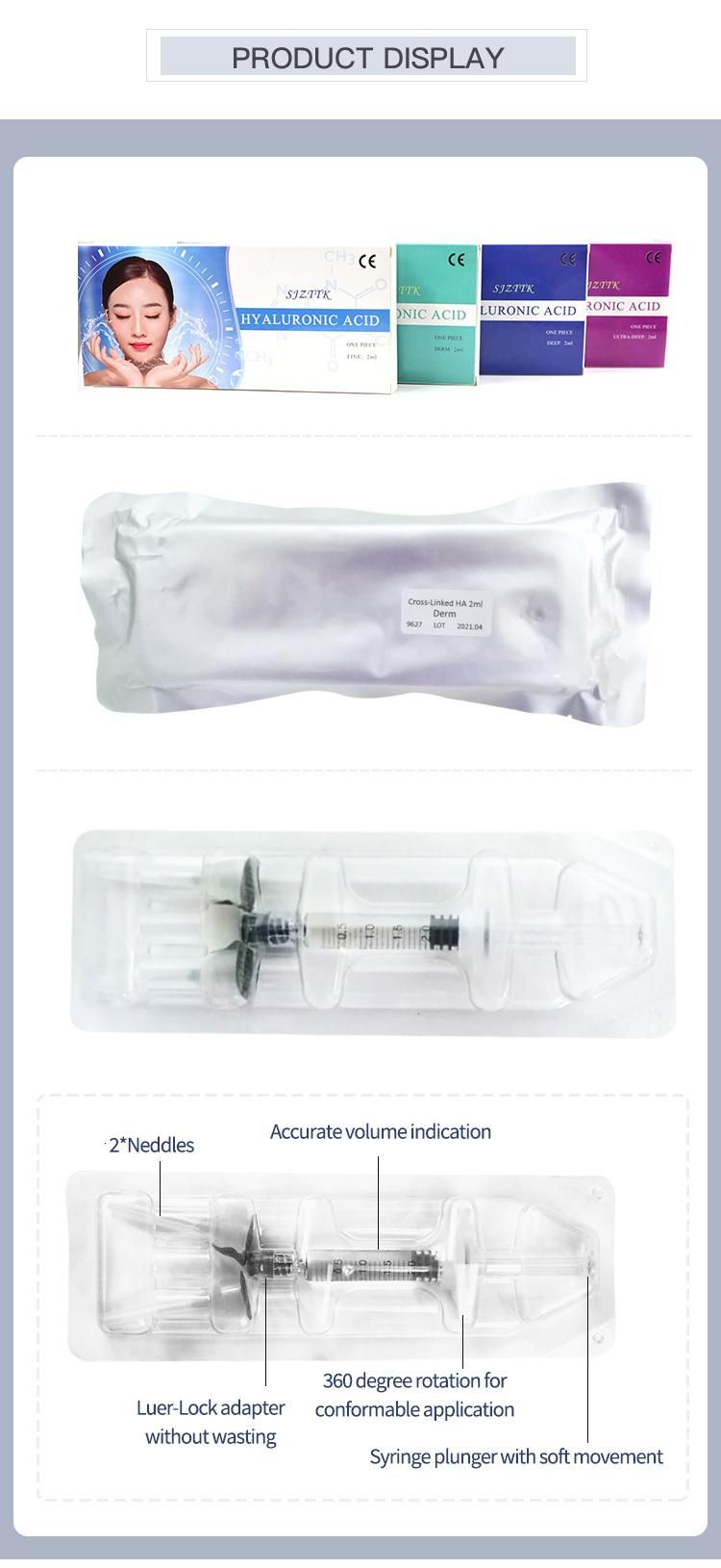
Hyaluronic acid (HA) fillers are used to plump up thin lines, deep expression wrinkles, and improve the global descent of the facial soft tissue. Typically, they are administered with a syringe or cannula and providers are instructed to discard any remaining filler left in the application device. However, fillers are expensive, so saving the leftovers for future touchups on the same patient would be helpful.A recent study examined the potential safety of saving and reusing HA fillers by evaluating the microbiological contamination of HA-based fillers stored for a long period after the original cosmetic use in patients. Using a protocol for storage designed for this study, the results showed that after a period of 44 days, no sample had any bacterial or fungal contamination.
The authors conclude that reuse of hyaluronic acid fillers, carefully stored after initial application, is a plausible option, with no increase in the risk of infections to the patient.

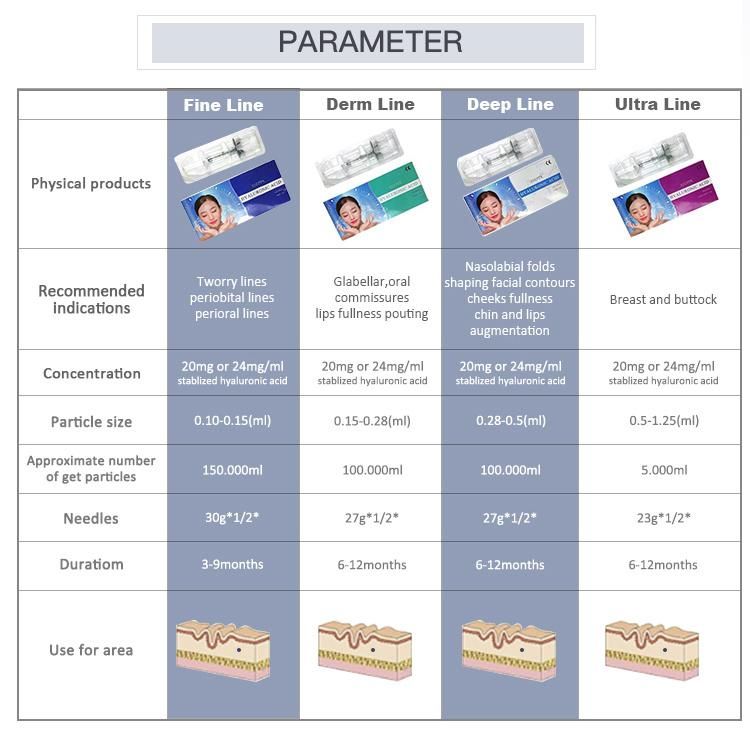


 Hyaluronic acid is an important substance in the human body to maintain moisture.It has
Hyaluronic acid is an important substance in the human body to maintain moisture.It has moisturizing factor, can effectively lock the skin moisture, enhance skin hydration function,
quickly penetrate into the bottom of the skin, deep hydration, promote skin absorption of nutrients,
smooth dry fine lines, improve relaxation, make skin moist and shiny at all times.

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





