ISO & CE Approved Epidural Kit (Type 2) for Single-Use
Shenzhen Xinhewei Plastic Packaging Co., Ltd. / 2022-06-23
- Type:Catheter
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:5 Years
- Group:Adult
- Logo Printing:Can Customized
=== Base Info ===
- Model NO.:MZTJ-3016
- Transport Package:100PCS,Carton, Can Do OEM
- Specification:CE, ISO
- Trademark:Huaxing Medical
- Origin:China
- HS Code:90189090
- Production Capacity:10000PCS,Month
=== Description ===
Basic Info.
Model NO. MZTJ-3016 Transport Package 100PCS/Carton, Can Do OEM Specification CE, ISO Trademark Huaxing Medical Origin China HS Code 90189090 Production Capacity 10000PCS/MonthProduct Description
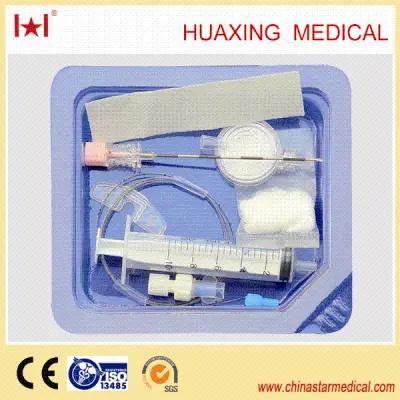
Disposable anesthesia epidural kit( anesthesia needle)Property:
1. For the anaesthesia, injection of epidural or lumbarspine
2. Single-Use to avoid cross infections
Specification:
Anesthesia kit I
Contents
1 no damage anesthesia catheter 1.0 or other 1
2 epidural needle 15G or 16G or other 1
3 catheter connector 1
4 air filter 1
5 thread assist guide 1
6 ordinary syringe 10ml 1
Anesthesia kit II
Contents
1 no damage anesthesia catheter 1.0 or other 1
2 epidural needle 15G or 16G or other 1
3 catheter connector 1
4 air filter 1
5 thread assist guide 1
6 ordinary syringe 10ml 1
7 loss of resistance syringe 10ml 1
Anesthesia kit III
Contents
1 no damage anesthesia catheter 1.0 or other 1
2 epidural needle 15G or 16G or other 1
3 catheter connector 1
4 air filter 1
5 thread assist guide 1
6 ordinary syringe 10ml 1
7 loss of resistance syringe 10ml 1
8 spinal needle 25G or other 1
9 anesthesia introducer needle 20G+22G+16G 1

New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





