PTFE/Hydrophilic Diagnostic Guidewire with ISO13485&CE Certification
Guangzhou Chenyang Package Materials Co, Ltd. / 2022-06-23

- Type:Surgical Supplies Materials
- Material:Nickeltitanium Wire Core
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
=== Base Info ===
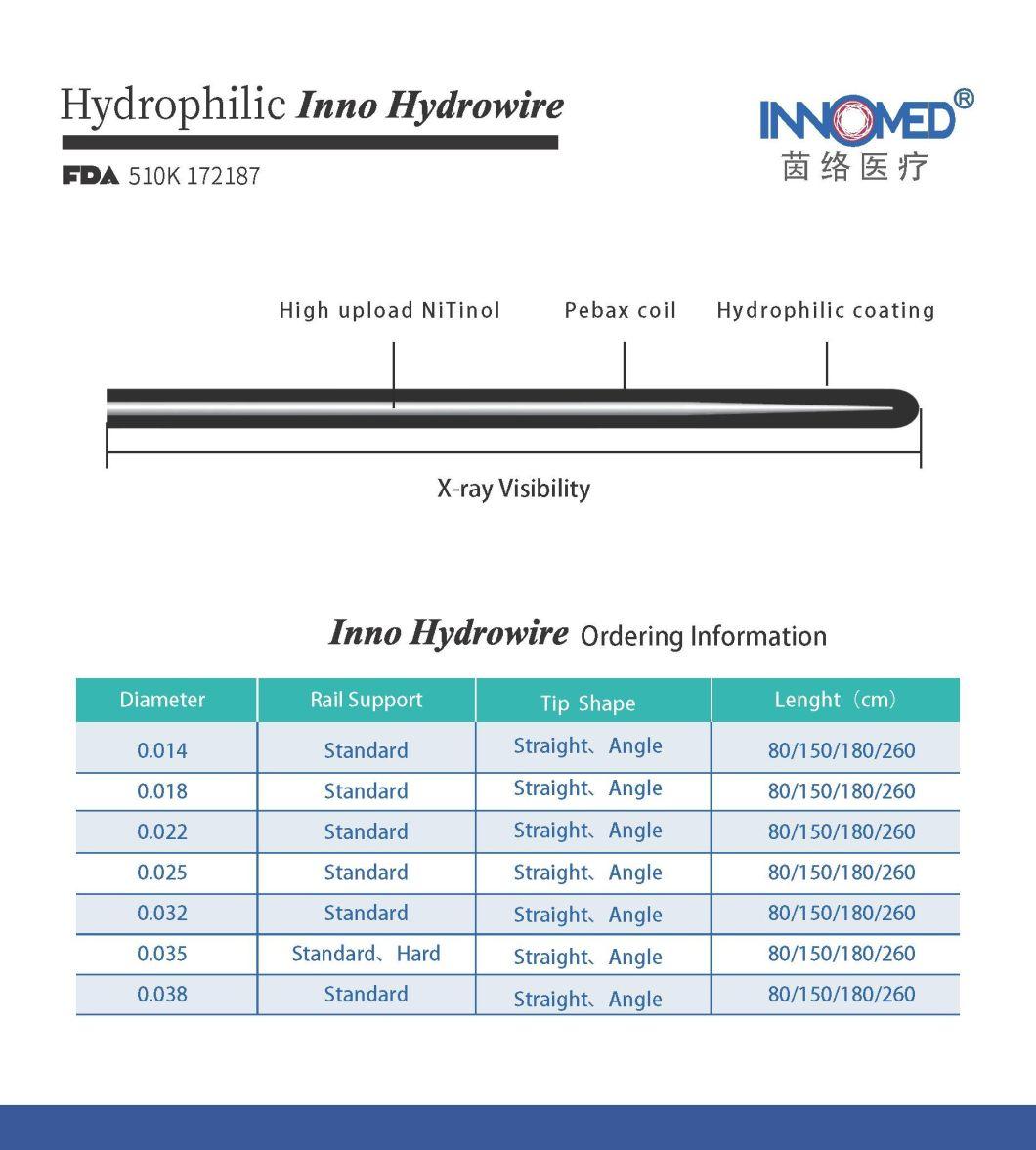
- Model NO.:Inno-Hydrowire
- Flex L:3cm
- Qty:5
- Stiffiness:Standard or Hard
- Tip Shape:Angle or Straight
- Certification:CE,ISO13485
- Transport Package:Cardboard Box
- Specification:SW-35-150-A-3-D
- Trademark:Innomed
- Origin:Suzhou China
- HS Code:9018909919
- Production Capacity:50000,Y
=== Description ===
Application range
It is mainly used for preoperative contrast diagnosis, guided contrast catheter, interventional therapy, guided balloon dilation catheter or stent.
Indications for use
The Inno-Hydrowire Guidewire is intended to direct a catheter to the desired anatomical location in the vascular system during diagnostic or interventional procedures. This device is not intended for neurovasculature or coronary vasculature.
Contraindications
The Inno-Hydrowire Guidewire is not intended for use in:
• The neurovasculature
• The coronary vasculature
• Patient judged not acceptable for percutaneous intervention
Warnings
• Do not manipulate or withdraw the guidewire through a metal needle or dilator.
• Do not advance or withdraw the device against resistance of unknown origin. Determine the cause by X-Ray and take necessary actions. Excessive force against resistance may result in vessel perforation /or damage.
• Do not attempt to use the guidewire if it has been bent, kinked or damaged.
• Do not put the guidewire into organic solvent, which may cause damage to the guidewire or its lubricity.
• Do not shape (reshape) the guidewire by any means. Reshaping the wire may cause damage and fracture.
• A torquer is included with guidewire, which is used to rotate and control the device. It cannot be inserted into the patient at any time.
• The guidewire is to be used by a physician who is thoroughly trained in the insertion, manipulation and observation of guidewires under fluoroscopy.
• The device should be disposed safely and properly, following local regulations and laws.
Precautions
• Physicians should evaluate the suitability of the device or select appropriate specifications of the device according to the patient's own condition and his/her own training skills and experience.
• The device must be moistened prior to removal from the dispenser in order to ensure lubricity. Heparinized physiological saline solution is recommended.
• Prior to reinsertion of the device during the same procedure, the wire should be rinsed in heparinized saline solution. Residual blood or other foreign matter can be removed by wiping the device with gauze that has been moistened with heparinized saline solution. Cleaning will not completely remove foreign substances, therefore the device is for SINGLE USE only.
Directions for use
1) Prepare the guidewire and any interfacing devices according to the manufacturer's directions.
2) Using sterile aseptic technique, remove the guidewire and the loop together from the package in the sterile field.
3) Using a syringe, flush the loop with heparinized saline solution through the tube of the loop to increase surface lubricity.
4) Remove the guidewire from the loop and inspect the guidewire prior to use; verify that it is lubricated.
NOTE: If the guidewire cannot be easily removed from the loop, inject more heparinized saline solution into the loop.
5) Prior to use, inject the catheter with heparinized physiological saline solution to ensure the guidewire can move easily within the catheter.
6) Tighten the torque at the proximal end of the wire and make sure there is no movement between them.
7) Keep at least 5cm of the tip of the wire out of the loop during introduction. Gently push and reverse the guidewire under fluoroscopy.
8) Holding the guidewire in position, advance the catheter over the guidewire and into the target lesion.
9) Complete the procedure and remove the guidewire and catheter according to standard procedural protocol.
10) Dispose of the guidewire according to your facilities hazardous waste policy.
Product Performance
Made of Nikle-titaniumalloy wire, it has good torsion control and support.
Visible under X-ray equipment, can accurately judge the moving status of guide wire.
The surface is coated with hydrophilic coating, which has good stability and increases the lubricity of the guide wire.
Product Line


Certificate


New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





