Human Parainfluenza Virus Type 3 Nucleic Acid Detection Kit
Brilliant Tin Box Manufacturing Co., Ltd. / 2022-06-23
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Classification:Biological Diagnostics
- Certification:CE, FDA, EEC, MSDS, ISO13485
- Transport Package:Carton
- Specification:58.5*58.8*62.5cm
=== Base Info ===
- Model NO.:SKY-8228
- Trademark:Runmei
- Origin:China
- HS Code:1108966068
- Production Capacity:10000boxes,Day
=== Description ===
Basic Info.
Model NO. SKY-8228 Trademark Runmei Origin China HS Code 1108966068 Production Capacity 10000boxes/DayProduct Description
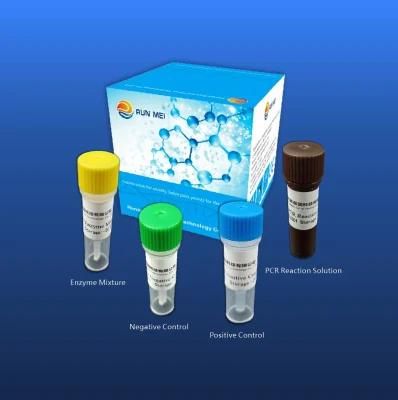
Human Parainfluenza Virus Type 3 Nucleic Acid Detection Kit(Fluorescent RT-PCR method)

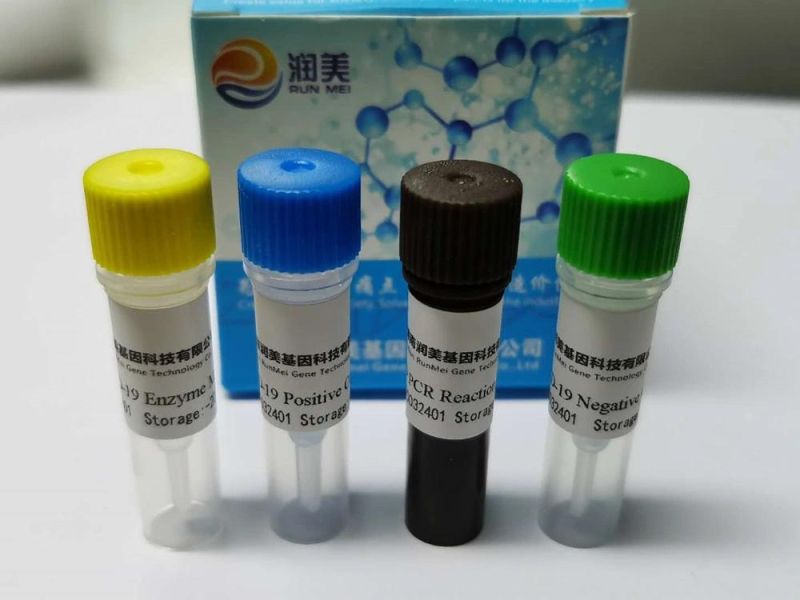

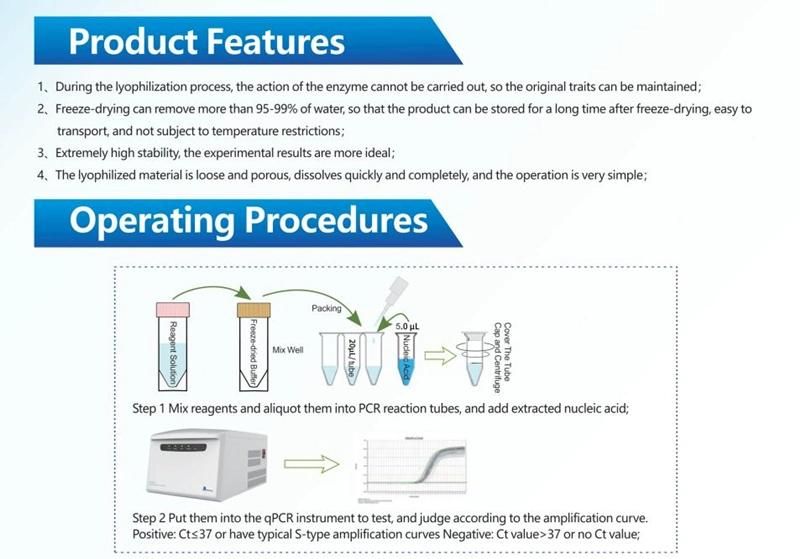

Company Profile:
to base on China and radiate the world, and to solve the pain points and difficulties of the industry and create value for human beings as our corporate purpose. At present, our company has completed the construction of product systems for pathogen biology fluoresence quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology immune colloidal gold detection kits,and disposable syringes.







New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





