Avian Influenza Virus Nucleic Acid Detection Kit
Brilliant Tin Box Manufacturing Co., Ltd. / 2022-06-23
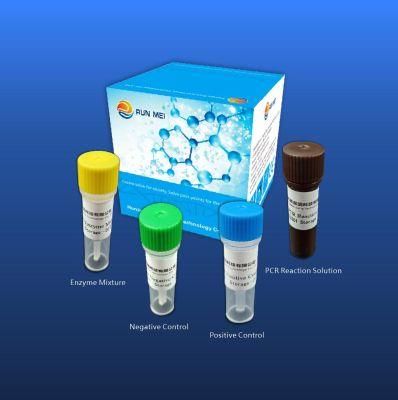
- Type:Avian Influenza Virus Nucleic Acid Detection Kit
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:One Year
- Group:Adult
- Logo Printing:With Logo Printing
- Transport Package:58.5*58.8*62.5cm
=== Base Info ===
- Model NO.:RM-6214
- Trademark:Runmei
- Origin:China
- Production Capacity:50000000 Per Month
=== Description ===
Basic Info.
Model NO. RM-6214 Trademark Runmei Origin China Production Capacity 50000000 Per MonthProduct Description
Avian influenza virus nucleic acid detection kit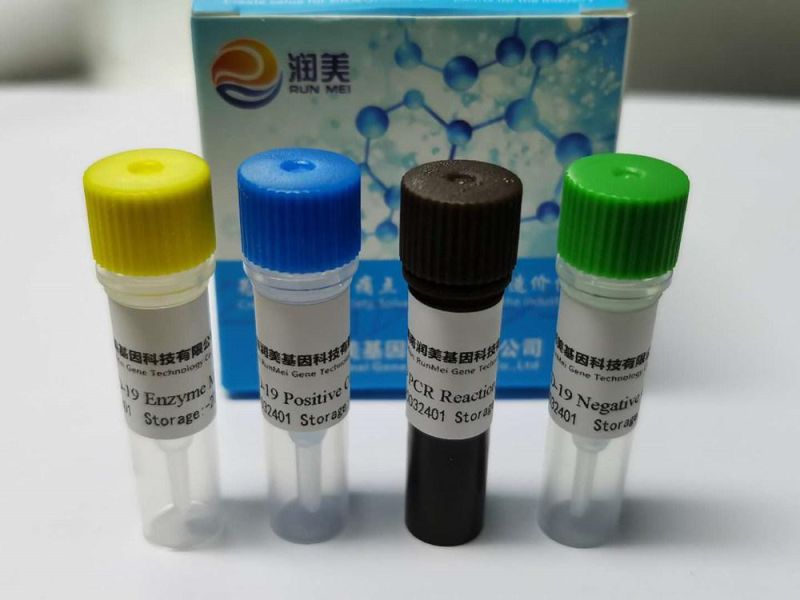


Immunoassay Series,Biochemical Series,Rapid Diagnosis Series reagent
Offer including Hepatitis A, B, C, E and STD, Runmei Gene IVD has new fist product categories as CCP (RA) and Tuberculosis, etc. HCV Core antigen kit and drug abuse tests are coming into the market
Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the development of the gene detection products and the construction of big data service platforms led by a team of doctors at home and abroad. Our company's strategic goal is to base on China and radiate the world, and to solve the pain points and difficulties of the industry and create value for human beings as our corporate purpose. At present, our company has completed the construction of product systems for pathogen biology fluoresence quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology immune colloidal gold detection kits. We are focus on IVD detection kits. We have our own R&D team, manufacture, many of them are professional doctors who have plenty of experience in this industry. Our products have approved many certificates like ISO, SGS,CE,CFDA and so on.




New product
Hot product
- Gm Candi Interface
2022-06-23
- Scan Dash V2.0 for BMW
2022-06-23
- K+Can Commander 2.0
2022-06-23
- Mut-3 Professional Diagnostic Tool Mut3
2022-06-23
- CKM-100 Key Programmer for Benz/BMW
2022-06-23
- Fuel Injector Tester & Cleaner (MST-N6A)
2022-06-23
- Mst 9000+ Plus ECU Simulator
2022-06-23
- OBD Ii Code Reader Mst-300, OBD2 Code Scanner
2022-06-23
- Digital Battery Analyzer (SC-100)
2022-06-23
- VAS5052A PC VERSION with VAS5054A Wireless Bluetooth Communicate
2022-06-23





